J.ophthalmol.(Ukraine).2022;6:39-43.
|
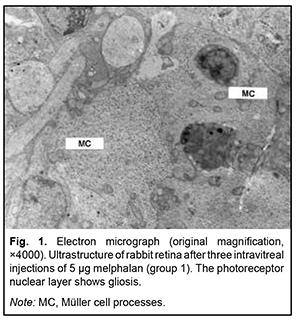
http://doi.org/10.31288/oftalmolzh202263943 Received: 19.10.2022; Accepted: 04.11.2022; Published on-line: 21.12.2022 Clinical and ultrastructural changes in the rabbit retina at various doses and numbers of intravitreal melphalan injections N. F. Bobrova, T. A. Sorochynska, N. I. Molchaniuk, O. Iu. Bratishko SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesа (Ukraine) TO CITE THIS ARTICLE: Bobrova NF, Sorochynska TA, Molachaniuk NI, Bratishko OIu. Clinical and ultrastuctural changes in the rabbit retina at various doses and numbers of intravitreal melphalan injections. J.ophthalmol.(Ukraine).2022;6:39-43. http://doi.org/10.31288/oftalmolzh202263943 Purpose: To assess clinical and ultrastructural changes in the rabbit retina and choroid with an increase in the dose and number of intravitreal (IVit) melphalan injections. Material and Methods: Thirteen eyes of seven Chinchilla rabbits (age, 5–6 months; weight, 2.5–3 kg) underwent a clinical electron microscopy (EM) examination. Rabbit eyes were divided into five groups based on the dose and number of IVit melphalan injections given. Group 1 (4 eyes) received three 5-μg IVit melphalan injections; group 2 (2 eyes), two 10-μg injections; group 3 (2 eyes), two 20-μg injections; group 4 (3 eyes), two 30-μg injections; and group 5 (4 eyes), two 40-μg injections. The interval between IVit melphalan injections was one month. Enucleation was performed at week 4 after the last injection. Results: In group 1, ophthalmoscopic changes in the retina (small isolated areas of mild depigmentation) stayed the same until the end of the observation period. On EM, some retinal pigment epithelium (RPE) cells showed signs of destruction, whereas others showed signs of compensatory-and-restorative processes. In addition, discs of the outer segments (OS) of photoreceptors showed pathological changes varying in severity. Other photoreceptor layers and retinal neural cells showed no ultrastructural changes. In group 2, fundus changes were more severe than in group 1, and somewhat increased in severity with each injection. There was EM evidence of significant pathology in RPE cells, extracellular edema in the photoreceptor layer, damage to the photoreceptor inner segments and OS, and focal necrosis of the neural tissue. In group 3, there was retinal pigment redistribution after the first injection, with an increase in the degree and area of depigmentation after subsequent injections. In addition, EM found RPE cells showing various pathological changes (e.g., as severe as destruction), pigment granules scattered throughout the retina, and retinal gliosis due to growth of hypertrophic Müller cell processes. In groups 4 and 5, there were large regions of marked depigmentation which increased in area with each injection, becoming almost confluent, which resulted in a complete discoloration of the retina. In addition, EM found necrosis of RPE cells and photoreceptors and RPE cell debris in the inner retinal layers, and the outer and inner retinal layers were lost and replaced by glia. Ganglion cells exhibited signs of degenerative changes. The choroid showed almost complete loss of choriocapillaries with signs of destruction of choroidal vascular walls. Conclusion: We demonstrated experimentally that repeated IVit melphalan injections at various doses did not affect media transparency and the structure of the anterior segment of the eye, and produced no general toxic effects on experimental animals. The clinical and ultrastructural changes in the retina depended on the dose of melphalan, with repeated 5-μg and 10-μg IVit melphalan injections being relatively safe and causing mild changes only in the outer retinal layers. Care should be taken when using IVit melphalan injections at hig (20-μg – 30-μg) doses, taking into account that toxic changes with various degrees of degeneration (e.g., as severe as gliosis of the retina and destruction of some choroidal capillaries) can develop. The purpose of the study was to assess clinical and ultrastructural changes in the rabbit retina and choroid with an increase in the dose and number of IVit melphalan injections. Material and Methods Thirteen eyes of seven Chinchilla rabbits (age, 5–6 months; weight, 2.5–3 kg) underwent a clinical and electron miscroscopy examination. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV dated 21.02.2006 and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. Rabbits were euthanized by air embolism under general anesthesia with sodium thiopental (1 ml/kg). Rabbit eyes were divided into five groups based on the dose and number of injections given. Group 1 (4 eyes) received three 5-μg IVit melphalan injections; group 2 (2 eyes), two 10-μg injections; group 3 (2 eyes), two 20-μg injections; group 4 (3 eyes), two 30-μg injections; and group 5 (4 eyes), two 40-μg injections. Intravitreal injection was performed in a standard manner [2]. The interval between intravitreal injections was one month. Eyes were examined at post-surgery days 1, 3 and 7, and at each week thereafter. Enucleation was performed at week 4 after the last injection. After enucleation, material for histology was taken in a routine manner [10, 11], and examined in a PEM-100-01 electron microscope. Results Clinical results IVit melphalan injections had no general toxic effect on the animals and resulted in no changes in the anterior eye segment and optic media. Initial ophthalmoscopic changes in the retina in the form of small isolated areas of mild depigmentation appeared 3 weeks after the second 5-μg IVit melphalan injection and these changes stayed the same until the end of the observation period. At 2 and 3 weeks after the third melphalan injection, there was an insubstantial increase in the degree, but not the area of depigmentation, with no depigmentation changes at subsequent time points. The changes in group 2 (two 10-μg injections) were similar to those in group 1, but fundus changes were more severe and somewhat increased in severity with each injection. Already after the first 20-μg melphalan injection, we noted pigment redistribution and the emergence of brighter and greater depigmentation regions, with an increase in the degree and area of depigmentation after subsequent IVit melphalan injections. In groups 4 and 5 (30- and 40-μg melphalan injections, respectively), there were substantial retinal changes with large regions of marked depigmentation after repeated injections. These regions increased in area with a rapid loss of pigment with each injection, becoming almost confluent, which resulted in a complete discoloration of the retina. No retinal hemorrhage, vitreous hemorrhage, retinal tear or detachment was observed at all follow-up points, regardless of how large an intravitreal dose of melphalan was administered. Ultrastructural changes Animals receiving repeated 5-μg melphalan injections showed mild, but more apparent ultrastructural changes than after a single 5-μg intravitreal injection: some retinal pigment epithelium (RPE) cells showed signs of destruction, whereas others showed signs of compensatory-and-restorative processes. The outer segment (OS) layer of photoreceptors showed regions of disrupted membrane discs and regions of focally-damaged discs, disc debris, or cell membrane ruptures. However, other portions of photoreceptors maintained their structure. Neural cells of subsequent retinal layers showed no changes. After the third injection, RPE cells showed various changes, with some of them exhibiting a loss of typical features, and others being more preserved; destroyed RPE cells appeared to be replaced by proliferative glial cells (Fig. 1). Neural cells of the inner retina appeared generally preserved, showing only focal cytoplasmic damage.
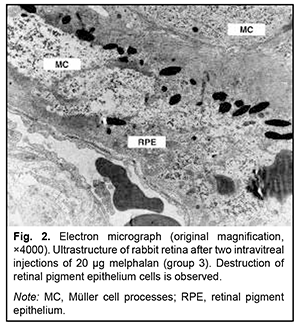
After two 10-μg IVit melphalan injections in group 2, the retinal layer structure appeared preserved. Hydropic changes were seen in the cytoplasm of RPE cells. The inner segment (IS) and OS layers of photoreceptors exhibited apparent extracellular edema. The photoreceptor nuclear layer appeared preserved. Cells of other retinal layers showed various pathological changes. Müller cells of the retina appeared hypertrophic, some of them exhibiting signs of destruction. Foci of necrosis of neural tissue were seen. The outer retinal layers were better preserved. Obviously, at that period, some retinal structures showed partial self-restoration. After two 20-μg IVit melphalan injections in group 3, scattered destroyed and semi-destroyed retinal structures were seen. The RPE cells showed various destructive changes, some of them appeared completely destroyed; pigment granules were scattered through the layers, Müller cells exhibited gliosis, and some glial cells appeared destroyed. Necrotic masses and isolated macrophages were frequently seen (Fig. 2).
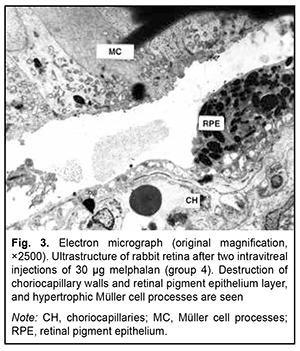
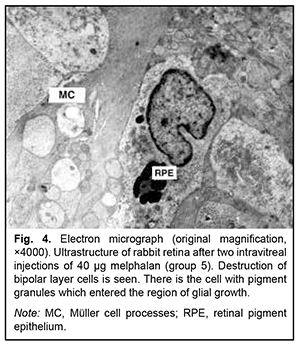
The retinal changes after repeated 30-μg melphalan injections in group 4 were similar to those after 40-μg melphalan injections in group 5, with almost complete loss of the RPE layer. Only fragments of the RPE layer and RPE cell debris were seen at some locations. Structurally changed RPE cells were seen in the inner retinal layers. Other types of retinal cells also showed various changes or destruction. The inner and outer segments of photoreceptors were lost and replaced by glia. Other outer segments of photoreceptors appeared penetrated by hypertrophic glial processes. Nerve fibers showed the most severe edema and destruction (Fig. 3, 4). Ganglion cells exhibited signs of degenerative changes, with the region of ganglion gliosis approaching the inner limiting membrane. The choroid showed almost complete loss of choriocapillaries with signs of destruction of the endothelium of choroidal vascular walls.
Therefore, our study demonstrated that the severity of ultrastructural changes in the rabbit retina after IVit melphalan injections depended on the size of the dose and the number of injections taken. The ultrastructural changes found in the retina conformed to the ophthalmoscopic changes in the fundus. Discussion Repeated 8-μg to 50-μg melphalan injections with an interval from 1 week to 1 month are used in the clinical setting. It was found that repeated small-dose melphalan injections did not cause marked complications and structural changes in the eye [6, 12], whereas 50-μg melphalan injections caused severe and expanding retinal atrophic lesions, hypotonia, retinal nerve atrophy and subatrophy of the eye [13]. Doses of 20 μg and 30 μg are the most commonly used doses of melphalan for local CHT [7, 14]. In a rabbit model developed by Inomata and Kaneko [15], a melphalan concentration of 5.9 μg/ml showed no retinal toxicity. In a light microscopy study by Ueda et al. (1995) [16], effects of an intravitreal injection of melphalan on the retinal structure were studied in rabbits to establish the non-toxic dose for its intravitreal use. The retinal structure remained unchanged after 10-μg injection, but moderately changed after 20-μg injection and greatly deteriorated after 90-μg injection. Shimoda et al. [17] studied the effect of intraocular melphalan perfusion at concentrations of 5-, 10-, and 20-μg/ml during pars plana vitrectomy on the rabbit retina. In the 5-μg/ml perfusion group, the histology showed no substantial changes compared with control fellow eyes during 28 days postoperatively. Histologic sections showed necrosis of the inner nuclear layer and thinning of the outer nuclear layer in the 10-μg/ml group. Loss of the outer nuclear layer and the IS/OS photoreceptor layer and necrosis of the inner nuclear layer were observed in the 20-μg/ml group. We have reported previously [10] that ultrastructural changes in the rabbit retina depended on the dose of a single IVit melphalan injection. There were no focal fundus changes in the eyes that received a single 5-μg IVit melphalan injection. In the eyes that received a single 10-μg IVit melphalan injection, there was focal destruction of RPE cells and photoreceptor outer segments. In the eyes that received a single 20-μg injection, there were disorganized retinal layers with loss of typical ultrastructural features of photoreceptors and retinal neurons, and Müller cell gliosis with focal destruction of their intracellular structures or necrosis [10]. In group 4 and group 5 eyes that received repeated 30-μg and 40-μg IVit melphalan injections in the current study, there was damage to all retinal layers and the choriocapillary layer of the choroid, with ganglion cells being more preserved, and destroyed retinal cells replaced by hypertrophic Müller cell processes. It is noteworthy that the literature is scant on experimental studies assessing the impact of various doses of intravitreal melphalan on the structure of the retina. In a study by Bar-Sela et al. [18], 18 rabbits were treated with IVit melphalan injections at a dose of 5 μg (group 1), 15 μg (group 2), 30 μg (group 3), or 60 μg (group 4), with an interval of 1 month, 2 weeks, 1 week and 3 days. No retinal changes were noted after a single 5-μg IV melphalan injection, and insubstantial (as much as 20%) thinning of the inner and outer nuclear layers was noted after repeated 5-μg IVit melphalan injections. Indirect ophthalmoscopy after injections revealed sclerotic retinal vessels and retinal whitening in the experimental eyes of groups 2 and 3. This was in line with destructive morphological changes like sclerotic and degenerative retinal changes, preserved Müller cells with glial fibrillary acidic protein, 21% and 44% thinning of the inner nuclear layer and 50% and 64% thinning of the outer nuclear layer. In group 4 (60-μg), there was disorganization of all retinal structures with disruption of the nuclear layers, indicating complete damage to these layers. In our point of view, this concentration is critical and unsafe, because our previous study [10] has demonstrated severe and marked atrophic processes in the retina in the rabbit eyes after large doses of melphalan. Our data on the dose-dependent retinal changes after single and repeated IVit melphalan injections are in agreement with the findings of foreign researchers. Our results, are, however, more precise and informative than theirs, because the rabbit retina was assessed not only by light microscopy, but also ultrastructurally, by electron microscopy, which allowed finding more subtle changes in all retinal layers irrespective of the dose and number of injections of melphalan. The changes we found after repeated IVit melphalan injections with various time intervals allow observing the longitudinal impact of repeated drug injections on the retina. Repeated 5-μg IVit melphalan injections were relatively safe and low toxic. Repeated 10-μg IVit melphalan injections caused changes in the outer retinal layers with focal cell necrosis, but the inner retinal layers were preserved due to their partial regeneration, with ophthalmoscopically normal retinal regions alternated with the regions of moderate degeneration. Repeated 20-μg and 30-μg IVit melphalan injections had a toxic effect on the retina, with increased severity and depth of damage to all the retinal layers: the intracellular structures appeared destroyed and affected by extensive gliosis, and necrotic masses and isolated macrophages were seen. These regions increased in area with a rapid loss of pigment with each injection, becoming almost confluent, which resulted in a complete discoloration of the retina. Therefore, we found that the main toxic effect of IVit melphalan injections is exerted on the retina and choroid, with an increase in the dose of injection resulting in an increase in severity and depth of damage to all the retinal layers. Retinal changes practically stayed the same until the end of the observation period after repeated 5-μg or 10-μg IVit melphalan injections. There were, however, irreversible degenerative changes in the retina after repeated 20-μg or 30-μg IVit melphalan injections. Conclusion First, we demonstrated experimentally that repeated IVit melphalan injections at various doses did not affect media transparency and the structures of the anterior segment of the eye, and produced no general toxic effects on experimental animals. Second, the dose-dependent clinical and ultrastructural changes in the retina, with repeated 5-μg and 10-μg IVit melphalan injections being relatively safe and causing mild changes only in the outer retinal layers. Finally, care should be taken when using IVit melphalan injections at larger (20-μg – 30-μg) doses, taking into account that toxic changes with various degrees of degeneration (e.g., as severe as gliosis of the retina and destruction of some choroidal capillaries) can develop.
References 1.Bobrova NF, Sorochinskaya TA. [Combined (intravitreal and intravenous) polychemotherapy in organ-preserving treatment for retinoblastoma]. Oftalmol Zh. 2011;2:38-44. Russian. 2.Bobrova NF, Sorochinskaya TA. [Intravitreal chemotherapy for retinoblastoma: our five-year experience]. Oftalmol Zh. 2015;3:59-68. Russian. 3.Bobrova NF, editor. [A monograph on retinoblastoma]. Odesa: Izdatelskii tsentr; 2020. Russian. 4.Bobrova NF, Sorochinskaya TA. Local retinoblastoma chemotherapy by intravitreal melphalan injections (the preliminary report). South-East European Journal of Ophthalmology. 2009; 2 (3-4): 28-34. 5.Francis JH, Brodie SE, Marr B, Zabor EC, Monde-sire-Crump I, Abramson DH. Efficacy and Toxicity of Intra- vitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology. 2017; Apr; 124(4): 488-495. 6.Kaneko A, Suzuki S. Eye-Preservation Treatment of Retinoblastoma with Vitreous Seeding. Jpn J Clin Oncol. 2003 Dec;33(12):601-7. 7.Munier FL, Gaillard MC, Soliman S, et al.Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012; 96(8): 1078–83. 8.Shah N, Pham D, Murray T, et al. Intravitreal and Subconjunctival Melphalan for Retinoblastoma in Transgenic Mice. J Ophthalmol. 2014;2014:829879. 9.Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, et al. Targeted retinoblastoma management: When to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. 2014 Sep;25:374–85. 10.Bobrova NF, Sorochynska TA, Molachaniuk NI, Bratishko AIu. Ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan. J Ophthalmol (Ukraine). 2020;4(495):50-5. 11.Reynolds ES. The use of lead citrate at high pH an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12. 12.Ghassemi F, Fahimeh Asadi Amoli F. Pathological findings in enucleated eyes after intravitreal melphalan injection. Int Ophthalmol. 2014 Jun;34(3):533-40. 13.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012; 130: 1268-1271. 14.Shields CL, Douglass AM, Beggache M, Say EA, Shields JA. Intravitreous chemotherapy for active vitreous seeding from retinoblastoma: Outcomes After 192 Consecutive Injections. The 2015 Howard Naquin Lecture. Retina. 2016 Jun; 36(6):1184-90. 15.Inomata M, Kaneko A. Chemosensitivity profiles of primary and cultured retinoblastoma cells in a human tumor clonogenic assay. Jpn J Cancer Res. 1987;78(8):858–68. 16.Ueda M, Tanabe J, Inomata M. et al. [Study on conservative treatment of retinoblastoma - effect of intravitreal injection of melphalan on the rabbit retina]. J Jpn Ophthalmol Soc. 1995;99:1230–35. Japanese. 17.Shimoda Y, Hamano R, Ishihara K, et al. Effects of intraocular irrigation with melphalan on rabbit retinas during vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008; 246(4):501-8. 18.Bar-Sela SM, Zayit-Soudry S, Massarweh A, et al. Retinal toxicity of intravitreal melphalan in albino rabbits. J Clin Exp Ophthalmol. 2018;9(1):707. DOI: 10.4172/2155-9570.1000707.
Disclosures Author contribution: All authors participated in the conception of the article and in writing the manuscript. The final version of the manuscript has been approved by all authors, who in turn are solely responsible for submitting the final version for publication. Conflict of interest statement: All authors have no real or potential conflict of interest (financial, personal, professional, or other interests) that could influence the subject matter or material described and discussed in this manuscript. Sources of support. There are no external sources of funding.
|