J.ophthalmol.(Ukraine).2022;6:19-23.
|
http://doi.org/10.31288/oftalmolzh202261923 Received:11.11.2022; Accepted: 08.12.2022; Published on-line: 21.12.2022 Prognostic risk factors for diabetic retinopathy in patients with type 2 diabetes mellitus I. S. Alifanov 1, 2, V. M. Sakovych 1 1 Dnipro State Medical University 2 Dnipropetrovsk Regional Clinical Ophthalmic Hospital Dnipro (Ukraine) TO CITE THIS ARTICLE: Alifanov IS, Sakovych VM. Prognostic risk factors for diabetic retinopathy in patients with type 2 diabetes mellitus. J.ophthalmol.(Ukraine).2022;6:19-23. http://doi.org/10.31288/oftalmolzh202261923
Purpose: To establish a relationship of diabetic retinopathy with the presence of lesions of other target organs, disease severity and the requirement for insulin therapy in patients with type 2 diabetes mellitus, and to determine the most significant prognostic markers. Material and Methods: We examined 270 patients (270 eyes) with type 2 diabetes mellitus. They were divided into the diabetic retinopathy group and no-diabetic retinopathy group. The status of diabetic target organs was determined as per endocrinologist’s, cardiologist’s, nephrologist’s, neurologist’s and vascular surgeon’s conclusions. Statistica v6.1 (Statsoft, Tulsa, OK) software was used for statistical analysis. The level of significance р < 0.05 was assumed. Results: Diabetic retinopathy positively correlated with severe diabetes (r = 0.383, p < 0.001), insulin therapy requirement (r = 0.389, p < 0.001), diabetic nephropathy (r = 0.350, p < 0.001), chronic kidney failure (r = 0.390, p < 0.001), and lower-extremity angiopathy (r = 0.312, p < 0.001). In addition, the risk of diabetic retinopathy was high in patients on insulin therapy (odds ratio (OR) 6.1; 95 % СІ 3.40-10.93), patients with diabetic nephropathy (OR 17.34; 95 % СІ 4.94-60.83), chronic kidney failure (OR 6.88; 95 % СІ 3.66-12.94), lower-extremity angiopathy (OR 19.15; 95 % СІ 4.24-86.45), coronary heart disease (OR 2.4; 95 % СІ 1.21-4.76) and essential hypertension (OR 4.29; 95 % СІ 1.22-15.10). The risk of diabetic retinopathy was higher in patients with severe diabetes (OR 5.79, 95% CI 3.26-10.26) as compared to patients with mild to moderate diabetes. Conclusion: The positive associations of diabetic retinopathy with chronic kidney failure, diabetic nephropathy and lower-extremity angiopathy were stronger than the positive associations with other diabetic target organs. In addition, there was a moderate positive correlation of diabetic retinopathy with severe diabetes and insulin therapy requirement. The association of diabetic retinopathy with acute coronary and cerebral events was not significant. Keywords: diabetes mellitus, diabetic retinopathy, target organs, prognostic markers
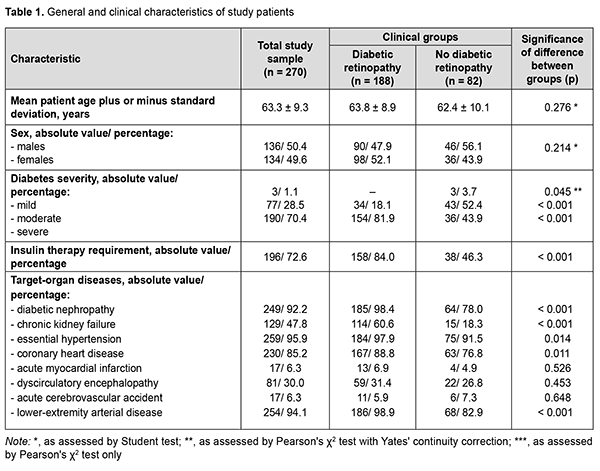
Introduction In 2021, globally, diabetes was estimated to affect 537 million adults (9.8% of the world’s population) aged 20–79 years and was responsible for 6.7 million deaths. The increase in global health expenditure due to diabetes has been considerable, growing from USD 232 billion in 2007 to USD 966 billion in 2021 for adults aged 20–79 years. This represents a 316% increase over 15 years. In 2021, in Ukraine, diabetes was estimated to affect 2.35 million adults and was responsible for 58.1 thousand deaths, and health expenditure due to diabetes was estimated at USD 1.45 million [1]. Modern medicine is equipped with an impressive arsenal of means for glycemic control and prolonged duration of life support, but vascular diabetic complications (including diabetic retinopathy, a specific retinal microvascular complication of diabetes that affects a third of people with diabetes) remain the major cause of disability and death among the people living with diabetes. It has been reported that 10% of patients will develop diabetic macular edema or proliferative diabetic retinopathy, a leading cause of blindness in adults in developed countries [2]. Patients with diabetic retinopathy reported reduced quality of life, difficulties in performing basic activities of daily living and professional activities, and reduced physical, emotional, and social well-being; a fifth of patients experienced difficulties in performing diabetes self-care management due to impaired vision [3]. In the most recent two decades, there has been an increase in the prevalence of diabetes in Ukraine (which is in line with global trends), with an increase in the level of accumulated disability due to diabetic retinopathy [4]. Improved understanding of diabetic retinopathy as a manifestation of the systemic disease facilitates the search for clinical and diagnostic markers, prediction of the development of severe ocular complications, and selecting the best strategy of treatment in patients with diabetes mellitus. Recent studies examined the global prevalence and major risk factors for diabetic retinopathy and vision-threatening diabetic retinopathy in people with diabetes and concluded that longer diabetes duration and poorer glycemic and blood pressure control are strongly associated with diabetic retinopathy [5, 6]. Given the fact that (1) diabetes mellitus results in generalized microvascular and macrovascular complications and (2) in vivo ophthalmoscopy of the fundus allows assessing the microcirculatory retinal bed, diabetic retinopathy is considered as a predictor and risk factor for the presence of lesions of other diabetic target organs like the cardiovascular system (including coronary syndrome [7, 8]), developing vascular neurological complications and cognitive impairment [9-11], and major lower-extremity angiopathy with a requirement for amputation [12, 13]. Therefore, we believe it feasible to determine somatic risk factors for diabetic retinopathy based on the analysis of the presence of lesions of other target organs in patients with diabetes mellitus. The purpose of the study was to establish a relationship of diabetic retinopathy with the presence of lesions of other target organs and the requirement for insulin therapy in patients with type 2 diabetes mellitus, and to determine the most significant prognostic markers. Material and Methods Two hundred and seventy patients (270 eyes) with type 2 diabetes mellitus who were treated on in-patient basis at the Endocrinological Department of Dnipropetrovsk Regional Clinical Hospital, and examined at the diabetic retinopathy room of Dnipropetrovsk Regional Clinical Eye Hospital, were included in this analytical prospective cohort study. Patients were divided into two groups based on the status of the worse eye as per the International Council Of Ophthalmology (ICO) criteria [2]: the diabetic retinopathy group (160 patients with non-proliferative retinopathy and 28 patients with proliferative retinopathy) and the no diabetic retinopathy group (82 patients including 46 men and 36 women). A general clinical eye examination (visual acuity, tonometry, and binocular ophthalmoscopy) and auxiliary eye examination (fundus photography, optical coherence tomography and ultrasound B scanning) were used to determine the presence of diabetic retinopathy. A Katena Diamond 90D non-contact lens and a Shin Nippon SL-45 slit lamp were used to perform fundus ophthalmoscopy, a Carl Zeiss Visucam 524 fundus camera, to perform fundus photography, an Optovue RT Vue 100-2 apparatus (Optovue Inc., Fremont, CA, USA) to perform spectral-domain optical coherence tomography, and a Quantel Medical Compact-II ultrasound system (Quantel medical, Clermont-Ferrand, France), to perform an ultrasound study. The status of diabetic target organs was determined as per endocrinologist’s, cardiologist’s, nephrologist’s, neurologist’s and vascular surgeon’s conclusions. Statistica v6.1 (Statsoft, Tulsa, OK; № AGAR909E415822FA) software was used for statistical analysis. Independent sample Student t‐test was used and mean and standard deviation (SD) values were calculated to compare normally distributed quantitative variables. Results were analyzed using contingency tables and Pearson Chi-Square test to test statistical group differences. Pearson's χ2 test with Yates' continuity correction was used for the difference values close to zero; otherwise, Pearson's χ2 test only was used. Spearman correlation was used to assess the relationship between characteristics. Associations of diabetic retinopathy with other diabetic systemic complications (reflected in odds ratio (OR) with 95% confidence intervals (CI)) were analyzed using univariate logistic regression and Wald test. The level of significance р < 0.05 was assumed. Results Mean patient age plus or minus SD was 63.8 ± 8.9 years for the DR group and 62.4 ± 10.1 years for the no-DR group (p > 0.05). There was also no significant difference in gender distribution between the groups (47.9 % male and 52.1% female for the former group and 56.1 % male and 43.9% female for the latter group (Table 1). Therefore, groups of the study were homogeneous for sex and age.
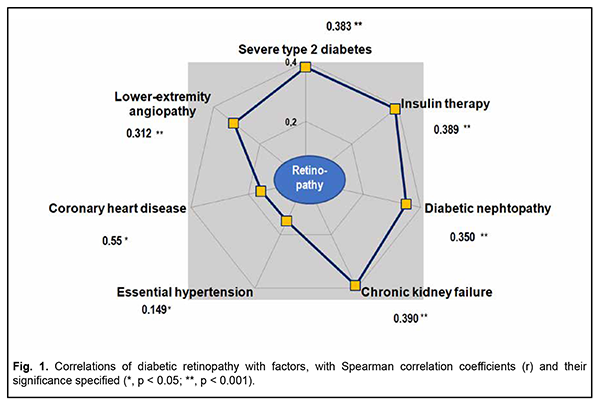
Severe disease was diagnosed in most (70.4%) patients with type 2 diabetes mellitus. In addition, the percentage of patients with severe diabetes in the DR group was almost twice higher than in the no-DR group (81.9 % versus 43.9 %, respectively, р < 0.001). Correspondingly, the percentage of patients requiring insulin therapy in the former group was higher (84.0 % versus 46.3 %, р < 0.001). Among the diseases of other target organs, essential hypertension was most common (259 patients; 95.9 %), followed by lower-extremity angiopathy (254 patients; 94.1 %), diabetic nephropathy (249 patients; 92.2 %), and coronary heart disease (230 patients; 85.2 %), with these diseases more commonly found in patients of the diabetic retinopathy group (р < 0.05 to р < 0.001). Chronic kidney failure was found in two thirds (60.6 %) of patients with DR and only in 18.3 % of patients without DR (р < 0.001). Such dangerous comorbidities of diabetes as acute myocardial infarction, acute cerebrovascular accident and dyscirculatory encephalopathy were uncommon (with their percentage incidences ranging from 6 to 30%), with no significant difference between the groups (p > 0.05). Clinically significant characteristics were selected and their prognostic potential for assessing the risk of diabetic retinopathy was determined based on the differences between the diabetic groups (with and without retinopathy) for the incidences of diseases of target organs. Correlation analysis confirmed that the most typical features of this diabetic complication of the eye were the presence of severe diabetes (r = 0.383, p < 0.001), insulin therapy requirement (r = 0.389, p < 0.001), diabetic nephropathy (r = 0.350, p < 0.001), chronic kidney failure (r = 0.390, p < 0.001), and lower-extremity angiopathy (r = 0.312, p < 0.001) (Fig. 1). There was a mild positive correlation of diabetic retinopathy to coronary heart disease (r = 0.155, p = 0.011) and essential hypertension (r = 0.149, p = 0.014).
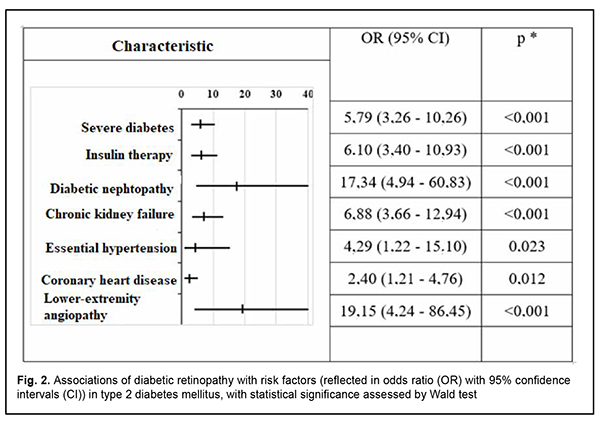
The risk of diabetic retinopathy was higher in patients with severe diabetes (OR 5.79, 95% CI 3.26-10.26) as compared to patients with mild to moderate diabetes (Fig. 2).
In addition, the risk of diabetic retinopathy was high in patients on insulin therapy (OR 6.1; 95 % СІ 3.40-10.93), patients with diabetic nephropathy (OR 17.34; 95 % СІ 4.94-60.83), chronic kidney failure (OR 6.88; 95 % СІ 3.66-12.94), lower-extremity angiopathy (OR 19.15; 95 % СІ 4.24-86.45), coronary heart disease (OR 2.4; 95 % СІ 1.21-4.76) and essential hypertension (OR 4.29; 95 % СІ 1.22-15.10). Discussion We found that there was a positive correlation of diabetic retinopathy with the presence of lesions of other target organs of type 2 diabetes. The positive associations of diabetic retinopathy with chronic kidney failure, diabetic nephropathy and lower-extremity angiopathy were stronger than the positive associations with other diabetic target organs. This generally confirms the concept of diabetes as a generalized microvascular disease, and is in agreement with the literature [7-13]. In addition, there was a moderate positive correlation of diabetic retinopathy with severe diabetes and insulin therapy requirement. The presence of diabetic nephropathy and lower-extremity angiopathy were found to be the strongest risk factors associated with diabetic retinopathy as assessed by odds ratios. It is noteworthy that these odds ratios were several times larger than those related to essential hypertension, coronary heart disease, insulin therapy requirement, etc. Of note was a disagreement between the correlation strength and odds ratio for the risk of developing diabetic retinopathy in patients with diabetic nephropathy and chronic kidney failure: the coefficient of Spearman correlation between diabetic retinopathy and chronic kidney failure was 0.390, whereas that between diabetic retinopathy and diabetic nephropathy was 0.350 (i.e., the former correlation was stronger), whereas odds ratio for the risk of the development of diabetic retinopathy were of 6.88 and 17.34, respectively. We believe that, given the fact that chronic kidney failure is a complication of diabetic nephropathy and develops only in patients with preexistent nephropathy, it is reasonable to consider a cumulative effect of odds ratios in this group of patients. Several foreign studies considered diabetic retinopathy as a risk factor and predictor of acute coronary and cerebral events [8, 9, 11, 14]; however, in the current study, the reverse association was not significant. In addition, no statistically significant association of the presence of diabetic retinopathy with age or sex was observed. Further research is planned to assess the diagnostic (prognostic) value of various predictors of diabetic retinopathy in patients with type 2 diabetes mellitus, and include the results of Doppler ultrasound of the ophthalmic artery in the study, given the fact that it is diabetic retinopathy that is a vascular manifestation of diabetes. In addition, we are going to determine the value of particular factors in the progression from non-proliferative to proliferative diabetic retinopathy, the latter bearing a high risk for visual disability and blindness in diabetics. The results obtained will contribute to advances in local clinical protocols and developing national guidelines for the treatment of diabetic retinopathy, clarification of indications for various therapeutic methods for ocular complications of diabetes mellitus.
References 1.International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://diabetesatlas.org/atlas/tenth-edition/ 2.International Council Of Ophthalmology. ICO Guidelines for Diabetic Eye Care (2017). Availble at: https://icoph.org/eye-care-delivery/diabetic-eye-care/ 3.Cavan D, Makaroff L, da Rocha Fernandes J, Sylvanowicz M et al. The Diabetic Retinopathy Barometer Study: Global perspectives on access to and experiences of diabetic retinopathy screening and treatment. Diabetes Res Clin Pract. 2017 Jul; Volume 129:16-24. 4.Alifanov IS, Sakovych VN, Alifanova TO. Disability due to ocular complications of diabetes mellitus in Ukraine. J Ophthalmol (Ukraine). 2019;6:34-8. 5.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. 6.Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36(6):1562-1568. 7.Brownrigg JR, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 2016;4(7):588-597. 8.Barlovic DP, Harjutsalo V, Gordin D, Kallio M, Forsblom C, King G, Groop P-H; on behalf of the Finn Diane Study Group, The Association of Severe Diabetic Retinopathy With Cardiovascular Outcomes in Long-standing Type 1 Diabetes: A Longitudinal Follow-up. Diabetes Care. 1 December 2018; 41 (12): 2487–94. 9.Hughes AD, Falaschetti E, Witt N, et al. Association of Retinopathy and Retinal Microvascular Abnormalities With Stroke and Cerebrovascular Disease. Stroke. 2016;47(11):2862-2864. 10.Hanff TC, Sharrett AR, Mosley TH, et al. Retinal microvascular abnormalities predict progression of brain microvascular disease: an atherosclerosis risk in communities magnetic resonance imaging study. Stroke. 2014;45(4):1012-1017. 11.Crosby-Nwaobi RR, Sivaprasad S, Amiel S, Forbes A. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36(10):3177-3186. 12.Foussard N, Saulnier P-J, Potier L, Ragot S, Schneider F, Gand E, et al.; on behalf of the SURDIAGENE Study Group, Relationship Between Diabetic Retinopathy Stages and Risk of Major Lower-Extremity Arterial Disease in Patients With Type 2 Diabetes. Diabetes Care 1 November 2020; 43 (11): 2751–2759. 13.Mohammedi K, Woodward M, Hirakawa Y, et al. Microvascular and Macrovascular Disease and Risk for Major Peripheral Arterial Disease in Patients With Type 2 Diabetes. Diabetes Care. 2016;39(10):1796-1803. 14.Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye diseaseand other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–78.
Disclosures Corresponding author: Alifanov I. S., alifanov00@gmail.com Disclaimer: The authors declare that the opinions expressed in this article are their own and not the official positions of the institution. Conflict of Interest. The authors declare that they have no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence, or be perceived to influence, their work. Sources of funding: none.
|