J.ophthalmol.(Ukraine).2022;6:65-69.
|
http://doi.org/10.31288/oftalmolzh202266569 Received: 31.10.2022; Accepted: 10.11.2022; Published on-line: 21.12.2022 A case of Susac’s syndrome: bilateral retinal vessel occlusion in the presence of autoimmune inflammatory endotheliopathy N. A. Ulianova 1, O. V. Zborovska 1, O. D. Shulga 2, 3 1 SI «The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine»; Odesa (Ukraine) 2 Volyn Regional Clinical Hospital; Lutsk (Ukraine) 3 Lesya Ukrainka Volyn National University; Lutsk (Ukraine) TO CITE THIS ARTICLE: Ulianova NA, Zborovska OV, Shulga OD. A case of Susac’s syndrome: bilateral retinal vessel occlusion in the presence of autoimmune inflammatory endotheliopathy. J.ophthalmol.(Ukraine).2022;6:65-69. http://doi.org/10.31288/oftalmolzh202266569
The paper presents a case with a classic triad (bilateral inferotemporal branch retinal artery occlusion (BRAO), multifocal callosal micro-infarcts, and sensorineural hearing loss) of Susac’s syndrome in a female patient following COVID-19 infection. We report clinical examination data, particularly, eye examination data and neurological status with brain magnetic resonance imaging (MRI) results. Special attention was given to the diagnostic value of optical coherence tomography (OCT) in Susac’s syndrome, which allows detecting characteristic focal atrophic changes in the inner retina with a preserved structure of the photoreceptor layer. Identification of the typical neurological, ocular or otological symptoms should raise suspicion for the syndrome, which is critical for early administration of the systemic steroid therapy. Keywords: Susac’s syndrome, autoimmune inflammatory endotheliopayhy, retinal artery occlusion
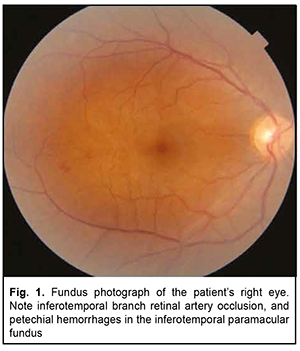
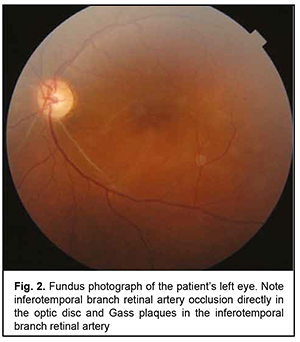
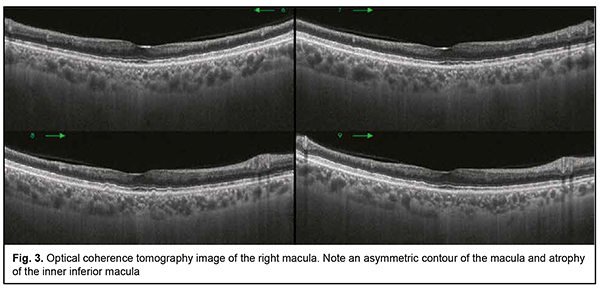
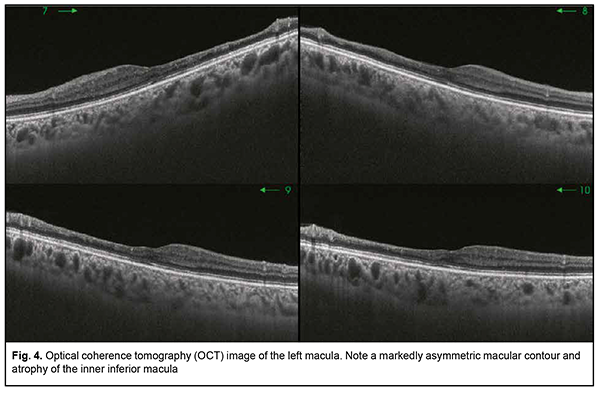
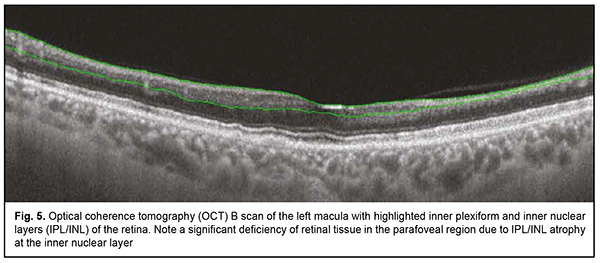
Introduction Susac’s syndrome, also known as small infarction of cochlear, retinal and encephalic tissue (SICRET) syndrome, is a microangiopathy which results in microvessel occlusion, with subsequent small infarcts in the brain, cochlea and retina, occurring mostly in young adult patients [1, 2]. The incidence of Susac’s syndrome is reported to be higher in females than in males. It is a rare condition whose definitive etiology remains unknown. At this time, it is classified as an immune-mediated, occlusive microvascular endotheliopathy and/or basement membranopathy that results in endothelium-induced microvascular occlusion in the central nerve system, inner ear, and retina [3]. There are limited reports on identifying and verifying the disease. Literature searches performed by Dörr and colleagues [4] in 2013, and by Vodopivec and Prasad [5] in 2017, yielded publications reporting 305 and 405 cases of Susac’s syndrome, respectively. More recently, the advent of modern techniques for imaging of retinal microinfarcts and brain tissue have led to an increase in the numbers of reports on detected cases [6, 7]. There may be a further increase in the number of patients with Susac’s syndrome following COVID-19 infection. Venditti and colleagues [8] reported the first case of Susac’s syndrome occurring after COVID-19 infection in a 25-year-old female. The disease was manifested by multiple acute and subacute ischaemic cortical strokes in the presence of increased serum anti-endothelial antibodies and in the absence of ocular or cochlear lesions. Ophthalmic manifestations (e.g., recurrent BRAOs), however, may be the sole presenting sign of Susac’s syndrome [6]. Most knowledge on the treatment Susac’s syndrome derives from the analysis of isolated reports and clinical experience, but there is no standardized treatment of the disease at this time. The disease is diagnosed on the basis of clinical findings and supporting evidence from examination of the eye, magnetic resonance imaging (MRI) of the brain, and audiometry. Therefore, the optimal diagnosis of this rare autoimmune disorder requires an integrated multidisciplinary approach involving ophthalmologists, neurologists and otorhinolaryngologists. Case report A 38-year-old female patient presented to the clinic and complained of an abrupt decrease in left visual acuity with the loss of the superior field of vision in her left eye two days before presentation. She also reported left headache and a decrease in hearing over a week before presentation. On eye examination, best-corrected visual acuity (BCVA) was 0.9 in the right eye and 0.3 in the left eye. Intraocular pressure (IOP) was 18 mmHg OD and 19 mmHg OS. Kinetic perimetry found superior visual field loss of 30 degrees and nasal visual field loss of 25 degrees in the left eye. Slit-lamp examination of the anterior eye and media was unremarkable bilaterally. Ophthalmoscopy findings included (a) partial BRAO of the inferotemporal arcade with isolated hemorrhages in the temporal macula and isolated Gass plaques in the right eye (Fig. 1) and (b) obliteration of the inferotemporal and inferonasal arcades, ischemic retina and ischemic inferior pole of the optic disc in the left eye (Fig. 2). Optical coherence tomography (OCT) findings included asymmetric contour of the macula with atrophic inferior inner macular regions, which was more apparent in the left eye; a central retinal thickness of 220 µm with moderate thinning of the inferior retina, in the right eye (Fig. 3); and a central retinal thickness of 196 µm with inferior retinal thinning as severe as 162 µm and 154 µm within 3-mm and 5-mm regions, respectively, in the left eye (Fig. 4). Specific changes in retinal OCT included focal inner retinal atrophy from the retinal neural fiber layer (RNFL) to the inner nuclear layer with relatively insubstantial changes in the thickness and structure of the outer retina. The inner retinal atrophy was most prominent in the areas of BRAO (Fig. 5), which corresponded to the inferior temporal fundus in both eyes. Given a symmetric pattern of retinal lesions, the patient received a preliminary diagnosis of Susac’s syndrome and was referred to a neuropathologist.
While examined by a neurologist, she complained of unilateral headache (mostly right side), usually in the temporal area and above the eye, over more than four hours, with intolerability of loud sounds, as frequent as 3-5 days a week, which was partially relieved with non-steroidal anti-inflammatory drugs; decreased memory for current events, decreased concentration, depression, and getting easily tired over the last three weeks. Her neurological status was remarkable for decreased left hearing, increased tendon reflexes, and intentional tremor while performing left-limb coordination tests. To exclude the presence of organic brain changes, the patient was referred for a brain MRI. Saggital T1-W scan showed sparse hypointense foci in the brainstem and splenium of the corpus callosum. Axial DWI scan showed numerous hyperintense foci in the body of the corpus callosum and periventricular white matter. Lumbar puncture was performed. The cerebrospinal fluid (CSF) was clear, and CSF examination showed protein 0.3 g/liter, lymphocytic cytosis (12 cells), and negative oligoclonal bands. The patient was consulted by an otorhinolaryngologist. Audiometry report revealed acute left sensorineural hearing loss. The patient was diagnosed with Susac’s syndrome on the basis of clinical findings and supporting evidence from MRI, audiogram and laboratory tests. She was administered methylprednisolone pulse therapy (1000 mg daily for 5 days), followed by prednisolone, starting with 50 mg daily, and reducing it by 5 mg every 5 days, for 7 weeks. Follow-up duration was 10 weeks. An improvement in the neurological status of the patient was noted over the follow-up. The BCVA improved to 1.0 in the right eye and 0.7 in the left eye. A superior nasal visual field loss of 20 degrees was still observed in both eyes. There was no change in audiometry with treatment, and left sensorineural hearing loss was still present. Discussion Susac’s syndrome was first described by Susac et al in 1979 as an unusual microangiopathy that affected the brain and retina in young adult females. At present, the syndrome is known as retinocochleocerebral vasculopathy characterized by the classic presentation with the clinical triad of subacute encephalopathy, BRAOs, and sensorineural hearing loss. It was designated as Susac’s syndrome by Hoyt in 1986 [9]. In this syndrome, isolated vessels show thickening and hyalinosis and endothelial cells become hypertrophic, which results in the occlusion of arterioles, leading to perivascular inflammation followed by ischemia of the brain and retina. Dorr and colleagues [4] compiled data from all 304 cases of Susac’s syndrome that were published worldwide till 2013, and found that only 13% of them had the characteristic clinical triad at disease onset. The assumption that presence of the triad is needed for a definite diagnosis of Susac’s syndrome is presumably a major cause of misdiagnosis [10]. The presence of only two components of the triad is interpreted as incomplete Susac’s syndrome. Therefore, the case reported here had the classical clinical triad of retinal vascular, brain and inner ear manifestations. Susac’s syndrome should be differentiated from autoimmune demyelinating disorders (multple sclerosis and acute disseminated encephalomyelitis), infectious encephalitis, primary and secondary central nervous system (CNS) vasculitides, Menière disease, toxic leukoencephalopathy, and Susac’s-like syndrome in chronic cocaine abusers [11]. In addition, the syndrome should be differentiated from autoimmune diseases (systemic lupus erythematosus and neural Behçet disease), paraneoplastic syndromes typical for patients on immunosuppressive medications or targeted tumor therapy [9]. The major differential diagnosis of Susac’s syndrome includes multple sclerosis and acute disseminated encephalomyelitis [12]. The white matter of the brain is most frequently affected in Susac’s syndrome, unlike demyelinating disorders, but the leptomeninges, grey matter, cerebellum and thalamus can also be affected. Involvement of the corpus callosum (in particular, MRI evidence of numerous hypointense “snowball lesions”) is considered a characteristic (pathognomonic) sign of Susac’s syndrome. In the case reported here, there was MRI evidence of three hypointense foci of microinfarct of the corpus callosum, and, therefore, MRI provided supportive evidence for the diagnosis of Susac’s syndrome. Lumbar puncture can reveal elevated protein levels in the cerebrospinal fluid with lymphocytic pleocytosis in Susac’s syndrome [13]. In the case reported here, lumbar puncture revealed elevated protein levels and lymphocytosis in the CSF. In addition, oligoclonal bands are important for the diagnosis of multiple sclerosis, and the CSF was negative for oligoclonal bands. The relative scarcity of the literature on Susac’s syndrome, a rare disease, may be partially explained by the fact that there are various non-classic presentations of the disease and the diagnosis of this condition is a challenge. Previous reports highlighted the value of ocular imaging techniques, retinal fluorescein angiography (FA) and funduscopy, in the diagnosis and treatment of Susac’s syndrome. Indeed, FA is an effective technique for detecting retinal circulation abnormalities. It was noted in a comprehensive review on Susac’s syndrome [4] that BRAO was found in 217 cases (99%) of the 219 cases in which the technique was used. The technique is, however, invasive and there are certain limitations for its use in clinical practice. Reduced visual functions in Susac’s syndrome are directly caused by atrophic changes in the retinal nerve fiber layer and ganglion cell layer as manifestations of microinfarcts due to inflammatory endotheliopathy. Consequently, when performing diagnostic assessment, it is crucial not only to establish the very fact of retinal vascular occlusion, but also to determine the severity of subsequent organic retinal changes. In this connection, the importance of OCT and OCT angiography (OCTA) in the diagnostic assessment of Susac’s syndrome has been highlighted recently, because pathological changes in the retinal vascular plexuses result in inner retinal abnormalities, whereas avascular outer retinal layers remain intact [14]. We did not use FA in the case reported here, because funduscopy and OCT showed specific retinal changes corresponding to signs of bilaterally symmetric previous inferotemporal BRAO, which enabled establishing a preliminary diagnosis of Susac’s syndrome and early patient referral to MRI and a neuropathologist. As a result, the diagnosis was confirmed and steroid therapy was early prescribed, and early administration of anti-inflammatory and immunosuppressive treatment has been demonstrated to result in good outcomes with recovery or stabilization of the condition. This treatment is a treatment of choice in patients with Susac’s syndrome. Conclusion The clinically complete Susac’s syndrome presents with a symptom complex involving the triad of pathognomonic symptoms of microinfarcts of retinal, brain and inner ear vessels due to autoimmune inflammatory endotheliopathy. Retinal OCT allows detecting specific ocular manifestations of Susac’s syndrome which present as focal atrophic changes in the inner retina in the presence of BRAOs. Manifestations of Susac’s syndrome in patients with a history of COVID-19 can be considered as a serious complication of the latter. Identification of the typical neurological, ocular or otological symptoms should raise suspicion for the syndrome, which is critical for early administration of systemic steroid therapy.
References 1.Kalisa P, Constantinides G, Bodson-Halleux M, De Laey JJ. Small infarctions of cochlear, retinal and encephalic tissue (SICRET syndrome). Bull Soc Belge Ophtalmol. 2001;282:5-12. 2.Szilasiová J, Klímová E. Susac syndrome: retinocochleocerebral vasculopathy. Croat Med J. 2004;45(3):338-43. 3.Al-Hasan Y, Hodkin JL, Wolf JC, Saha K. Susac Syndrome and Pregnancy. Case Rep Neurol Med. 2020 Dec 23;2020:6049126. 4.Dörr J, Krautwald S, Wildemann B, Jarius S, Ringelstein M, Duning T, Aktas O, Ringelstein EB, Friedemann P, Kleffner l. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013 Jun;9(6):307-16. 5.Vodopivec I, Prasad S. Short follow-up bias confounds estimates of the “typical” clinical course of susac syndrome. J Neuroophthalmol. 2017 Jun;37(2):149–53. 6.Sauma J, Rivera D, Wu A, Donate-Lopez J, Gallego-Pinazo R, Chilov M, Wu M, Wu L. Susac’s syndrome: an update. Br J Ophthalmol. 2020 Sep;104(9):1190-1195. 7.Redler Y, Chwalisz BK. Neuro-ophthalmic manifestations of Susac syndrome. Curr Opin Ophthalmol. 2020 Nov;31(6):495-502. 8.Venditti L, Rousseau A, Ancelet C, Papo T, Denier C. Susac syndrome following COVID-19 infection. Acta Neurol Belg. 2021 Jun;121(3):807-809. 9.Vishnevskia-Dai V, Chapman J, Sheinfeld R, et al. Susac syndrome: clinical characteristics, clinical classification, and long-term prognosis. Medicine (Baltimore). 2016;95(43):e5223. 10.Kleffner I, Duning T, Lohmann H, et al. A brief review of Susac syndrome. J Neurol Sci. 2012;322(1–2):35–40. 11.Hantson P, Di Fazio V, Del Mar Ramirez Fernandez M, et al. Susac-Like Syndrome in a Chronic Cocaine Abuser: Could Levamisole Play a Role? J Med Toxicol. 2015;Vol. 11(1):124–128. 12.Susac JO. Susac’s Syndrome. Am J Neuroradiol. 2004;25:351–352. 13.Pereira S, Vieira B, Maio T, Moreira J, Sampaio F. Susac's Syndrome: An Updated Review. Neuroophthalmology. 2020 May 1;44(6):355-360. 14.Azevedo AGB, Lima LH, Muller L, et al. Anatomical and functional correlation in Susac syndrome: multimodal imaging assessment. Int J Retina Vitreous. 2017;3:39.
Disclosures Corresponding author: Ulianova N. A., e-mail: ulyanova@ukr.net Author Contributions: Ulianova N.A.: Conceptualization, Writing – original draft, Determining the patient’s ophthalmological status; Zborovska O.V.: Conceptualization, Writing – original draft, Performing the Differential Diagnosis; Shulga O.D.: Writing – original draft, Determining the Patient’s Neurological Status, Magnet Resonance Imaging. All authors reviewed the results and approved the final version of the manuscript. Disclaimer. The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions. Ethical statement. Informed consent was obtained from the participant of the study. Financial support. No financial support was received for this work. Conflict of Interest Statement. No author reports any relevant conflicts of interest.
|