J.ophthalmol.(Ukraine).2022;6:3-9.
|
http://doi.org/10.31288/oftalmolzh2022639 Received: 25.08.2022; Accepted: 21.11.2022; Published on-line: 21.12.2022 Value of morphological OCT changes in the posterior lens capsule in optimization of surgical treatment for posterior capsular cataracts N. S. Lutsenko 1, 2, O. A. Isakova 1, 2, O. A. Rudycheva 1, 2, T. S. Kyrylova 1, 2, G. V. Iatsun 2 1 State Institution “Zaporizhia Medical Academy of Post-Graduate Education Ministry of Health of Ukraine” 2 Zaporizhzhia Regional Clinical Hospital Zaporizhzhia (Ukraine) TO CITE THIS ARTICLE: Lutsenko NS, Isakova OA, Rudycheva OA, Kyrylova TS, Iatsun GV. Value of morphological OCT changes in the posterior lens capsule in optimization of surgical treatment for posterior capsular cataracts. J.ophthalmol.(Ukraine).2022;6:3-9. http://doi.org/10.31288/oftalmolzh2022639 Background: It is reasonable and important to optimize the diagnostic and surgical measures for faster visual rehabilitation of patients with posterior capsular cataract (PCC). Purpose: To investigate morphological changes (as assessed by optical coherence tomography (OCT)) in the posterior lens capsule (PLC) in PCC, and to assess their value in the optimization of phacoemulsification for these cataracts. Material and Methods: Of the 1780 eyes (1200 patients) with cataract examined, 512 eyes (28.8%) were diagnosed with PCC. Patients had OCT of the anterior segment (AS-OCT) and phacoemulsification. The morphological changes in the PLC as assessed by OCT, the course of surgical treatment and perioperative complications were reviewed. Results: Three types of morphological changes in the PLC were identified. Type 1 changes were characterized by a clear PLC margin and found in 312 of the 512 eyes (61%). A posterior capsule-sparing phacoemulsification (i.e., with low power, vacuum and irrigation settings) was used only in 28% of eyes. In type 2 changes, the PLC margin was not uniform in reflectivity and thickness, but appeared clear at the retrolenticular space. Type 2 changes were found in 185 of the 512 eyes (36%). We refrained from hydrodissection in 95.1% of cases, used a posterior capsule-sparing phacoemulsification in 97% of cases and posterior capsule polishing in all cases of PLC with type 2 changes. Type 3 changes were characterized by PLC bulging into the retrolenticular space, and were found in 15 eyes (3%). A capsule rupture was observed in 53% of eyes with type 3 changes, although we used a posterior capsule-sparing phacoemulsification with viscodilation in these cases. Conclusion: AS-OCT allows detailed assessment of morphological changes in the PLC and identification of three types of these changes. It is reasonable to take in account these facts when selecting a surgical treatment strategy. Keywords: posterior capsular cataract, optical coherence tomography
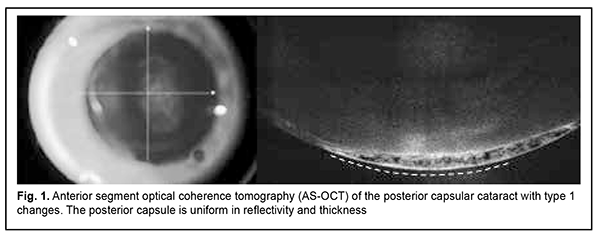
Introduction Cataract surgery is one of the most efficient methods of treatment in the history of medicine, and its hardware, instruments and techniques have been continuously improved in recent decades. It is this that contributes to a prompt restoration of visual functions in cataract patients and enables them going back to their routine daily activities and restoring their quality of life [1]. Planning for and decision making in phacoemulsification (phaco) is a comprehensive and responsible activity aimed at improving the efficacy and safety of the method with regard to surgical complications. This is especially related to non-standard or complicated cases involving a small-pupil syndrome, pseudoexfoliation (exfoliation syndrome), ocular hypertension or lens subluxation. The surgical techniques for and preventive measures against these pathological conditions have been widely reported in the literature [2, 3, 4]. This improves the surgeon’s confidence and readiness for timely decision making in unexpected situations during phaco surgery. In addition, the type of cataract (nuclear, cortical, subcapsular, posterior capsular, etc.) at baseline should be taken into account, because each type has certain features and is characterized by certain pathological lens changes which can influence treatment time and complication development [5, 6]. However, the presence of changes in the posterior subcapsular layers and posterior capsule do not always receive proper focus in routine daily practice. Cases may differ in the cause of the development and pathogenesis of lens opacity in the posterior capsular cataract (PCC). PCC can be either congenital or acquired. Age-related lens changes can mask the clinical appearance of congenital PCCs in older patients, whereas acquired PCCs usually form in the presence of changes in the body, systemic diseases (long-time administration of steroids), or eye disease (history of vitrectomy, etc.) [7, 8, 9]. PCCs result in a more substantial vision loss already in early disease stages and are more frequently found in young individuals compared to other types of cataracts. In addition, studies have reported that cumulative 5-year progression rates and incidences of surgery were higher for PCC than for other types of cataract [5, 10]. Therefore, it is reasonable to optimize the diagnostic and surgical activities for faster visual rehabilitation of patients with PCC. PCC surgery can be challenging due to various clinical situations. Therefore, it is important to comprehensively assess all changes in the morphology of the anterior and posterior segments of the eye before surgery, in order to timely use the departures from the stages of phaco, and thus facilitate the avoidance of surgical complications. It is this problem that the paper seeks to address. The purpose of the study was to investigate morphological OCT changes in the posterior lens capsule in posterior capsular cataract, and to assess their value in the optimization of phaco for these cataracts. Material and Methods This study was conducted from 2020 until 2022, and 1200 patients (1780 eyes) requiring cataract surgery were examined within the framework of the study. Five hundred and twelve (28.8%) of 1780 eyes were diagnosed with PCC. Informed consent was obtained from all patients participating in the study. The study was approved by the local ethics committee and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Preoperative best-corrected visual acuity (BCVA) ranged from 0.02 to 0.3 (Sivtsev chart). Preoperatively, a general eye examination (BCVA, perimetry, tonometry, biomicroscopy, ophthalmoscopy, and ocular biometry) and biochemical tests were conducted as per the relevant protocols. Optical coherence tomography (OCT) was conducted in eyes with PCC to identify morphological changes in the anterior and posterior segments of the eye, and study groups were formed based on the changes identified in the eyes. OCT was performed in mydriasis with a Optovue RTVue 100 XR Avanti (Optovue Inc, Fremont, CA) using Line and CrossLine scans (high-definition B scans), and 3D Cornea scans. We performed horizontal and vertical scans focusing on the posterior capsule of the lens and showing also the core of the lens and the retrolenticular space. A portion of the lens nucleus and the anterior hyaloid membrane were also seen in some of the scans performed. Additional scans focusing on the anterior capsule of the lens were performed in case of changes in the most anterior lens. 3D Cornea scans made it possible to examine a 4x4 mm field and capture the entire or most of the opacity. OCT images of the posterior lens capsule and opacity were analyzed, the relationship of the posterior lens capsule and lens opacity was examined and the state of the retrolenticular space was assessed. If the major opacity was adjacent to the posterior capsule and the latter was difficult to assess, we tried to improve the visualization of better reflective structures. With this in mind, contrast settings were changed, allowing enhancing the visualization of denser structures and assessing the capsule margin more accurately. Each OCT image initially obtained with standard OCT settings was assessed by two independent experts. If there was a discrepancy between the two experts, “hyperdiagnosis” (i.e., predicting worse morphological changes in the lens) was given an advantage. Surgery was performed by one experienced surgeon. This facilitated (1) more accurate distribution among the groups based on the morphological changes in the lens, and (2) the prevention of perioperative iratrogenic complications. Surgery consisted of multimodal anesthesia and ultrasound phaco combined with artificial lens implantation. For phacoemulsification, the Alcon Infiniti Vision System (Alcon, Fort Worth, TX, USA) was used in conjunction with a 45-degree Kelman 0.9-mm mini-flared tip. The efficacy measure was the number of perioperative complications for each of the morphological types. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Continuous variables are presented as mean ± standard error of mean (SEM), and categorical variables are presented as percentages. Results There was no significant difference in diagnostic biomicroscopy characteristics of pathological lens changes between cases (eyes), i.e., in each case, there was opacity of various severities and areas in the posterior cortical lens, the rest of the lens being clear. However, anterior segment (AS)-OCT and posterior segment OCT facilitated detailed understanding of the morphological changes and allowed identifying their characteristic features and forming several study groups. Thus, group 1 was most numerous and comprised 61% (312) of the study eyes. Morphological changes in the posterior lens capsule (PLC) in this group were defined as type 1 PLC changes and characterized by the presence of a sharp margin along the posterior capsule. The capsule margin was practically uniform in reflectivity and thickness. The layers adjacent to the posterior capsule were not uniform in reflectivity and showed hyporeflective inclusions, cracks and homogenous hyperreflective foci in the posterior cortical layers (Fig. 1).
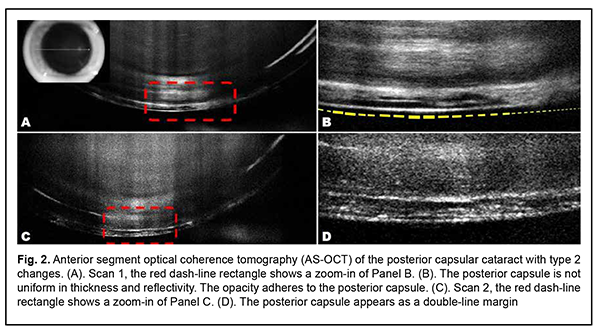
In group 2 comprising 36% (185) of the study eyes, the margin of the posterior capsule was not uniform in reflectivity and thickness and appeared as a double line. In addition, it appeared clear at the retrolenticular space. A hyporeflective focus in the cortical layer was flush with the posterior capsule thus making it thicker, and at some sites of the focus, it was impossible to delineate the capsule. These changes in the PLC were defined as type 2 changes (Fig. 2).
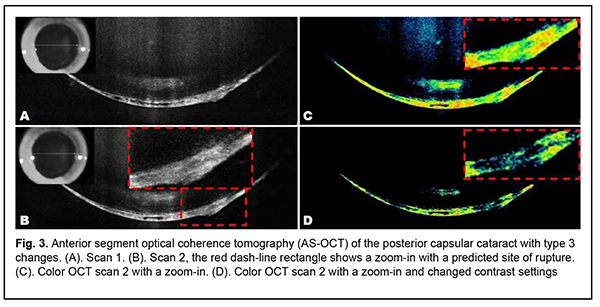
In group 3 comprising 3% (15) of the study eyes, the posterior capsule was either not clearly visualized due to hyperreflective lesion or bulged into the retrolenticular space (indicating a possible defect in or disintegration of the posterior capsule) and there was a fibrous cord. These changes in the PLC were defined as type 3 morphological changes (Fig. 3).
The distribution of patients by the type of morphological changes in the posterior lens capsule is shown in Table 1.
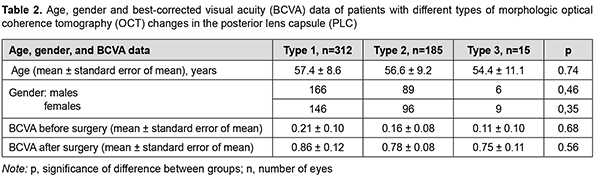
The use of contrast-enhanced AS-OCT improved visualization of the posterior capsule and resulted in a change in the numbers of patients in study groups compared to baseline (Table 1), and expert opinions became more consistent. The number of eyes in group 1 increased by 13.8% compared to baseline, from 274 to 312. Totally, the AS-OCT allowed clarifying the morphological lens changes in 7.4% of eyes. Although there was no change in the number of eyes in group 3 compared to baseline, qualitative changes were identified in three eyes which showed potential damage to the posterior capsule initially. It was confirmed that there was no disintegration of the posterior capsule in one of the three eyes (Fig. 1). Data on patient age, gender, and preoperative and postoperative BCVA were calculated for each type of morphological changes in PCC (Table 2).
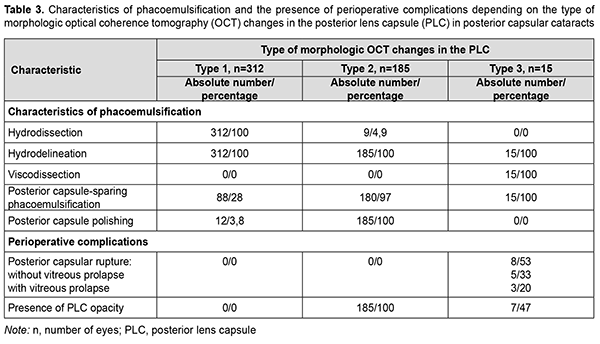
Morphological OCT changes in PCC did not depend on patient’s demographic data (Table 2). The posterior capsular cataracts received surgical treatment which depended on the identified OCT structural changes in the PCC and involved some departures from the standardized phaco stages. Characteristics of the phaco techniques and relevant perioperative complications were summarized for the identified types of morphological changes in PCC (Table 3).
Hydrodissection, hydrodelineation and ordinary phaco were performed in all cases with type 1 morphological AS-OCT changes (i.e., no AS-OCT evidence of close interrelationship of the posterior capsule with the surrounding structures). The nucleus and subcapsular layers were removed sparing the capsule using low irrigation and aspiration flow to reduce the pressure on the capsule and, consequently, to preserve capsular integrity, only in 88 eyes (28%). Additional polishing of the posterior capsule was used in 12 eyes (3.8%). As a result of sequential measures employed, no perioperative complications were observed. A strong attachment of the posterior cortical layers to an altered lens capsule in cases with type 2 morphological OCT changes gave grounds for the following measures: refraining from hydrodissection in 95.1% of cases and performing complete hydrodelineation in all cases; and using low irrigation and aspiration flow to remove the nucleus in 97% of cases and posterior capsule polishing in all cases. This facilitated avoiding perioperative complications. However, posterior capsular changes in the form of opacification regions of various opacity severity score and area were noted in all cases, which required postoperative laser treatment. Because type 3 morphological OCT changes in PLC indicate potential loss of posterior capsular support (i.e., degeneration or loss of integrity of the PLC), preserving the PLC during phacoemulsification was a challenge. In some patients, viscodissection was used to carefully separate the peripheral lens cortex and epinucleus from the compromised capsule. In addition, contrary to cases with type 2 morphological OCT changes in PLC, posterior capsule polishing was not used because we believed that it was not reasonable and unsafe given the state of the capsule in cases with type 3 morphological OCT changes. Moreover, posterior capsular rupture was seen in half of cases with type 3 morphological OCT changes (particularly, posterior capsular rupture without vitreous prolapse was seen in 33%, and surgery was combined with vitrectomy in 20% of cases), and the rest showed opacification, which required laser discision. Therefore, a preoperative understanding of AS-OCT morphological changes in PLC substantially facilitates planning PCC surgery and avoiding potential perioperative complications. Discussion Intracapsular complications in the form of posterior capsular tear and partial loss of vitreous are common in patients undergoing PCC surgery. Osher and colleagues [11] and Vasavada and Singh [12] performed phacoemulsification on posterior polar cataracts and reported on a posterior capsular rupture rate of 26% and 36%, respectively. In a study by Hayashi and colleagues [13], medical records of 28 eyes of 20 consecutive patients with posterior polar cataract who had cataract surgery were reviewed. Of the 28 eyes, 25 (89.3%) with a small to medium posterior polar opacity had standard phacoemulsification or aspiration surgery. Among the eyes having phacoemulsification or aspiration surgery, posterior capsule rupture occurred in 2 (7.1%). In the current study, we paid attention to the features of lens opacities, optimized phaco stages in PCC surgery, and found an intraoperative complication rate of 1.5%, with half of intraoperative complications related to type 3 morphological OCT changes in PLC. In order to reduce the rate of posterior capsular rupture, others have given attention to (1) diagnostic measures aiming to identify the features of lens opacities which can cause such complications and (2) optimization of surgical cataract removal. Kumar and colleagues [14] used biomicroscopy to study the effect of size of the posterior polar opacity on surgical outcome of phacoemulsification in posterior polar cataract. Consecutive patients with posterior polar cataract who underwent phacoemulsification were analyzed for intraoperative complications. The rate of intraoperative posterior capsule rupture was higher in eyes with polar opacities 4 mm or more than in eyes with less than 4 mm size (30.43% versus 5.71%, respectively). Kumar and colleagues concluded that phacoemulsification in eyes with larger size of polar opacity has significant risk of posterior capsule rupture. Guo and colleagues [15] were the first to report the application of 25MHz B-Scan ultrasonography (MHzB) to distinguish between the integrity and non-integrity (dehiscence) of the posterior capsule in posterior polar cataract. The authors concluded that the results of the study could be used to select the appropriate treatment and to thereby avoid further complications during posterior polar cataract surgery. Since 2014, there have been reports on the use of AS-OCT for (1) determining whether the posterior capsule is intact or there is a pre-existing posterior capsule defect or (2) assessing the adhesion of the capsule to the posterior polar opacity and subsequently determining the eyes at high risk for posterior capsule rupture (i.e., perioperative complications) [16, 17, 18]. In the current study, we also used AS-OCT, but, contrary to previous relevant AS-OCT studies, changed contrast settings to exclude less reflective morphological structures. This significantly improved our understanding of the morphological changes in the lens (particularly, (1) the state, integrity and transparency of the posterior capsule, or (2) opacity location, relation with the capsule, density and extension, and (3) the state of the retrolenticular space and the posterior hyaloid membrane) in patients with PCC. In addition to the advances in the diagnostic methods that facilitate determining the risks of posterior capsular rupture, and, consequently, reducing the rate of perioperative complications in patients with PCC, there have been advances in the surgical methods with the same purpose. Various techniques and preventive measures for minimizing the risk of posterior capsular rupture during phaco in patients with PCC have been reported. First, this is related to hydrodissection and hydrodelineation stages, to be exact, to careful separation of the opacity proper from an altered posterior capsule. Some authors believe that, in order to avoid hydraulic rupture of the capsule, hydrodissection should not be performed [12, 19]. Fine and colleagues [20], however, performed hydrodissection in multiple quadrants and gently injected tiny amounts of fluid such that the fluid wave could not extend across the posterior capsule. The next stage of phaco, hydrodelineation for creating a mechanical cushion of the epinucleus, can be also performed in various ways. With conventional hydrodelineation, the cannula penetrates the lenticular substance and thus causes the fluid to traverse from the outside inward. It is sometimes difficult to introduce the cannula within a firm nucleus, and the effort can rock and stress the capsular bag and zonules. The surgeon may also inadvertently inject fluid into the subcapsular plane and thereby conduct unwarranted hydrodissection [21]. Inside-out delineation [22] was proposed by Vasavada and Rai, is easy to perform and enables nucleus separation without additional risks, but requires additional cannulas. Allen and Wooda and Taskapili and colleagues [23, 24] reported on the feasibility of viscodissection for gentle dissecting only the peripheral cortex from the capsule. Second, nucleus removal requires sparing the capsule with low power, vacuum and irrigation flow settings to reduce the pressure on the capsule [23, 25]. In our practice, we differentially used various PCC removal techniques depending on the morphological OCT changes in the PLC. Thus, phaco was conducted using a conventional technique in group 1. Multiple-quadrant hydrodissection and hydrodelineation were performed in group 2. In group 3, we mostly used a combination of hydrodelineation and viscodelineation, which facilitated efficient phaco and IOL implantation. Capsule-sparing phacoemulsification was used in all the cases which had been found by AS-OCT to have alterations in the posterior capsule. Therefore, visual rehabilitation success in patients with PCC depends both on adequate preoperative examination and adequate surgical treatment. AS-OCT enables assessing morphological changes in the lens, whereas a new technological solution like a change in contrast settings for AS-OCT imaging of the posterior capsule enables a more detailed understanding of the state of the capsule and its interrelationships with the surrounding structures. A specific surgical treatment strategy should be chosen for a patient with PCC taking into account the developed morphological changes in the lens. This paper is a part of the research program entitled Optimization of Eye Disease Diagnosis, Treatment and Follow-up with Ocular Coherence Tomography and Angiography (registration number № 0119U101932).
References 1.de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction [Internet]. Vol. 2016, Cochrane Database of Systematic Reviews. 2016 Dec 12 [cited 2022 Aug 10];12(12):CD003169. Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003169.pub4/... 2.Yang S, Jiang H, Nie K, Feng L, Fan W. Effect of capsular tension ring implantation on capsular stability after phacoemulsification in patients with weak zonules: a randomized controlled trial. CTR implantation in cataract patients with weak zonules. BMC Ophthalmol [Internet]. 2021 Jan 7 [cited 2022 Aug 10];21(1):1–11. Available from: https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-020-01772-8 3.Yang Y, Chen H, An J, Fan W. Long-term effects of phacoemulsification and intraocular lens implantation in a patient with pathologic myopia and extremely long axial length: A case report. Medicine (Baltimore) [Internet]. 2020 Sep 11 [cited 2022 Aug 10];99(37):e22081. Available from: https://journals.lww.com/md-journal/Fulltext/2020/09110/Long_term_effect... 4.Fontana L, Coassin M, Iovieno A, Moramarco A, Cimino L. Cataract surgery in patients with pseudoexfoliation syndrome: current updates. Clin Ophthalmol [Internet]. 2017 Jul 31 [cited 2022 Aug 10];11:1377–83. Available from: https://www.dovepress.com/cataract-surgery-in-patients-with-pseudoexfoli... 5.Panchapakesan J, Mitchell P, Tumuluri K, Rochtchina E, Foran S, Cumming RG. Five year incidence of cataract surgery: the Blue Mountains Eye Study. Br J Ophthalmol [Internet]. 2003 Feb 1 [cited 2022 May 15];87(2):168–72. Available from: https://pubmed.ncbi.nlm.nih.gov/12543745/ 6.Chan E, Omar F, Mahroo AR, Bchir MB, Spalton DJ, Frcp F, et al. Complications of cataract surgery. Clin Exp Optom [Internet]. 2010 Nov 1 [cited 2022 Mar 19];93(6):379–89. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1444-0938.2010.00516.x 7.Feng H, Aadelman RA. Cataract formation following vitreoretinal procedures. Clin Ophthalmol [Internet]. 2014 Sep 23 [cited 2022 Mar 19];8:1957–65. Available from: https://www.dovepress.com/cataract-formation-following-vitreoretinal-pro... 8.Richardson RB, Ainsbury EA, Prescott CR, Lovicu FJ. Etiology of posterior subcapsular cataracts based on a review of risk factors including aging, diabetes, and ionizing radiation. Int J Radiat Biol [Internet]. 2020 Nov 1 [cited 2022 Mar 19];96(11):1339–61. Available from: https://www.tandfonline.com/doi/abs/10.1080/09553002.2020.1812759 9.Rim THT, Kim MH, Kim WC, Kim TI, Kim EK. Cataract subtype risk factors identified from the Korea National Health and Nutrition Examination survey 2008-2010. BMC Ophthalmol [Internet]. 2014 Jan 10 [cited 2022 May 9];14(1):1–15. Available from: https://bmcophthalmol.biomedcentral.com/articles/10.1186/1471-2415-14-4 10.Klein BEK, Klein R, Moss SE. Incident cataract surgery: The Beaver Adam Eye Study. Ophthalmology. 1997;104(4):573–80. 11.Osher RH, Yu BCY, Koch DD. Posterior polar cataracts: a predisposition to intraoperative posterior capsular rupture. J Cataract Refract Surg [Internet]. 1990 [cited 2022 Aug 10];16(2):157–62. Available from: https://pubmed.ncbi.nlm.nih.gov/2329471/ 12.Vasavada A, Singh R. Phacoemulsification in eyes with posterior polar cataract. J Cataract Refract Surg [Internet]. 1999 Feb[cited 2022 Aug 10];25(2):238–45. Available from: https://pubmed.ncbi.nlm.nih.gov/9951671/ 13.Hayashi K, Hayashi H, Nakao F, Hayashi F. Outcomes of surgery for posterior polar cataract. J Cataract Refract Surg [Internet]. 2003 Jan 1 [cited 2022 Aug 10];29(1):45–9. Available from: https://pubmed.ncbi.nlm.nih.gov/12551666/ 14.Kumar S, Ram J, Sukhija J, Severia S. Phacoemulsification in posterior polar cataract: does size of lens opacity affect surgical outcome? Clin Experiment Ophthalmol [Internet]. 2010 Dec 1 [cited 2022 Aug 10];38(9):857–61. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1442-9071.2010.02354.x 15.Guo Y, Lu C, Wu B, Gao J, Li J, Yuan X, et al. Application of 25MHz B-Scan Ultrasonography to Determine the Integrity of the Posterior Capsule in Posterior Polar Cataract. J Ophthalmol [Internet]. 2018 [cited 2022 Aug 10];23:1–5. Available from: https://pubmed.ncbi.nlm.nih.gov/29785304/ 16.Chan TCY, Li EYM, Yau JCY. Application of anterior segment optical coherence tomography to identify eyes with posterior polar cataract at high risk for posterior capsule rupture. J Cataract Refract Surg. 2014 Dec 1;40(12):2076–81. 17.Kymionis GD, Diakonis VF, Liakopoulos DA, Tsoulnaras KI, Klados NE, Pallikaris IG. Anterior segment optical coherence tomography for demonstrating posterior capsular rent in posterior polar cataract. Clin Ophthalmol [Internet]. 2014 Jan 10 [cited 2022 Mar 19];8:215–7. Available from: https://www.dovepress.com/anterior-segment-optical-coherence-tomography-... 18.Dhami A, Dhami N, Dhami S. Role of ASOCT in Preoperative Assessment of Posterior Polar Cataract. Acta Sci Ophthalmol. 2020;3:2582–3191. 19.Siatiri H, Moghimi S. Posterior polar cataract: minimizing risk of posterior capsule rupture. Eye [Internet]. 2005 Oct 28 [cited 2022 Aug 10];20(7):814–6. Available from: https://www.nature.com/articles/6702023 20.Fine IH, Packer M, Hoffman RS. Management of posterior polar cataract. J Cataract Refract Surg. 2003 Jan 1;29(1):16–9. 21.Vasavada AR, Raj SM, Vasavada V, Shrivastav S. Surgical approaches to posterior polar cataract: a review. Eye [Internet]. 2012 Mar 23 [cited 2022 Apr 19];26(6):761–70. Available from: https://www.nature.com/articles/eye201233 22.Vasavada AR, Raj SM. Inside-out delineation. J Cataract Refract Surg [Internet]. 2004 Jun [cited 2022 Aug 10];30(6):1167–9. Available from: https://pubmed.ncbi.nlm.nih.gov/15177589/ 23.Allen D, Wooda C. Minimizing risk to the capsule during surgery for posterior polar cataract. J Cataract Refract Surg [Internet]. 2002 [cited 2022 Aug 10];28(5):742–4. Available from: https://pubmed.ncbi.nlm.nih.gov/11978449/ 24.Taskapili M, Gulkilik G, Kocabora MS, Ozsutcu M. Phacoemulsification with viscodissection in posterior polar cataract: minimizing risk of posterior capsule tear. Ann Ophthalmol (Skokie) [Internet]. 2007 Jun [cited 2022 Aug 10];39(2):145–9. Available from: https://pubmed.ncbi.nlm.nih.gov/17984504/ 25.Salahuddin A. Inverse horse-shoe technique for the phacoemulsification of posterior polar cataract. Can J Ophthalmol. 2010;45(2):154–6.
Disclosures Corresponding Author: Rudycheva O. A., e-mail: rudychevaolga5@gmail.com Author Contributions: Lutsenko NS: Conceptualization, Supervision, Investigation, Data Analysis and Interpretation, Writing – original draft, Writing – review & editing; Isakova OA: Investigation, Data Analysis and Interpretation, Formal Analysis, Writing – original draft, Writing – review & editing; Rudycheva OA: Methodology, Investigation, Data Analysis and Interpretation, Writing – review & editing; Kyrylova TS: Methodology, Investigation, Data Analysis and Interpretation, Writing – review & editing; Iatsun GV: Resources, Investigation, Writing – review & editing. All authors reviewed the results and approved the final version of the manuscript. Disclaimer: The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions. This paper is a part of the research program entitled Optimization of Eye Disease Diagnosis, Treatment and Follow-up with Ocular Coherence Tomography and Angiography (registration number № 0119U101932). Conflict of Interest Statement. The authors state that they have no conflict of interest that might bias this work. Ethical Statement: This study included human participants, was approved by the local bioethics committee and adhered to the tenets of the Declaration of Helsinki. Appropriate informed consent was obtained. This study did not include animal experiments. Abbreviations: AS-OCT, anterior segment optical coherence tomography; PCC, posterior capsular cataract; BCVA, best-corrected visual acuity; OCT, optical coherence tomography; Phaco, phacoemulsification of cataract.
|