J.ophthalmol.(Ukraine).2021;2:30-36.
|
http://doi.org/10.31288/oftalmolzh202253036 Received: 07.09.2022; Accepted: 06.10.2022; Published on-line: 27.10.2022 A novel technique of evisceration or enucleation secondary to trauma, chronic uveitis or uveal melanoma, with permanent and removable fixation of the ocular prosthesis in a musculoskeletal stump A. P. Maletskyi 1, N. M. Bigun 2 1 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine); Odesa (Ukraine) 2 Lviv Regional Clinical Hospital; Lviv (Ukraine) TO CITE THIS ARTICLE: Maletskyi AP, Bigun NM. A novel technique of evisceration or enucleation secondary to trauma, chronic uveitis or uveal melanoma, with permanent and removable fixation of the ocular prosthesis in a musculoskeletal stump. J.ophthalmol.(Ukraine).2022;5:30-6. http://doi.org/10.31288/oftalmolzh202253036
Background: The state and motility of a cosmetic ocular prosthesis are important. Purpose: To develop a novel technique of evisceration or enucleation secondary to trauma, chronic uveitis or uveal melanoma, with permanent and removable fixation of the ocular prosthesis in a musculoskeletal stump (MS). Material and Methods: Group 1 comprised 52 patients with chronic uveitis secondary to trauma and phthisis bulbi who underwent evisceration. After evisceration, a polymer composite implant or a polytetrafluoroethylene (PTFE) implant was used to shape an MS with a hole in it for the pegged prosthesis, and the prosthesis motility in these patients was compared with that in the 13 controls in whom an MS without a hole in it for the pegged prosthesis was shaped. Group 2 comprised 31 patients with uveal melanoma who underwent enucleation with a PTFE implant used to shape an MS with a hole in it. The prosthesis motility in these patients was measured and compared with that in the 100 controls in whom an MS without a hole in it for the pegged prosthesis was shaped. Results: In patients of group 1, total prosthesis motility at 3 and 12 months improved to 132.50 ± 6.40 and 147.30 ± 6.70, respectively, versus 103.70 ± 18.30 and 103.10 ± 6.00, respectively, in the controls. No implant exposure was observed over the follow-up period. In three patients of group 2, diastasis of the conjunctival margins with implant exposure was observed at the margin of the hole at months 3 and 7, which necessitated implant removal. In patients of group 2, total prosthesis motility in the four meridians at 3 and 12 months was 141.60 ± 14.70 and 142.20 ± 16.10, respectively, versus 106.10 ± 13.00 and 103.70 ± 18.30, respectively, in the controls. Conclusion: We found that firm fixation of the pegged ocular prosthesis in the MS allowed improving total prosthesis motility in the four meridians at 3 months and 12 months after evisceration, by 28.8о and 44.2о, respectively, and at 3 months and 12 months after enucleation, by 35.5о and 38.5о, respectively. Keywords: PTFE implants, polymer composite implants, musculoskeletal stump, trauma, uveitis, uveal melanoma
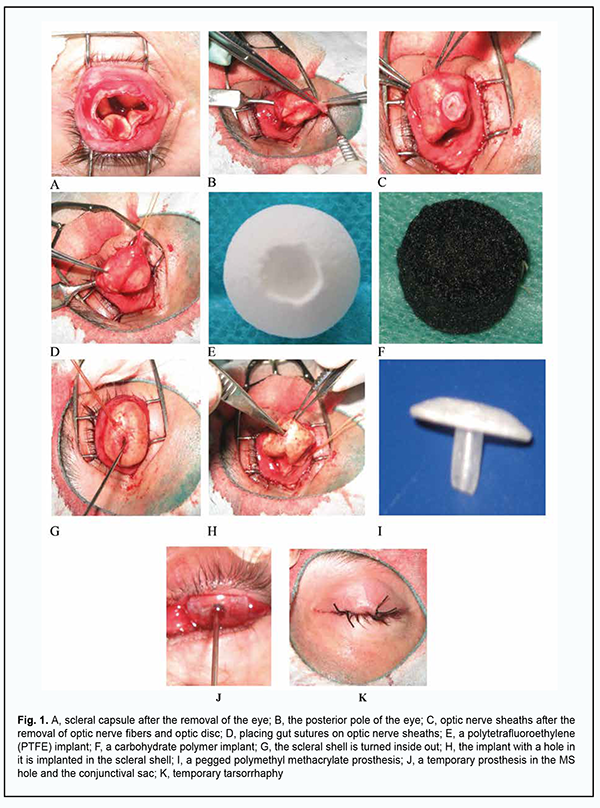
Introduction Craniofacial injuries represent 29% of all trauma cases [1-3] and are primarily caused by anthropogenic and criminal-related ocular and orbital trauma [4]. It has been reported that 11.6% to 27.0% of patients with penetrating trauma undergo enucleation or evisceration [5, 6]. In 2005, the annual number of these procedures performed in Ukraine was 2520 [5]. Intraocular malignancies are another cause for enucleation. In 2006 through 2010, the rate of enucleation for uveal melanoma at the Filatov institute was 56% [7], which is in agreement with the data (50.11%) reported by the Collaborative Ocular Melanoma Study [8]. Problems of cosmetic ocular prosthesis are important, and solving these problems will facilitate social and professional rehabilitation of individuals who underwent enucleation of the globe [9]. The ophthalmic surgeon has to use implant materials to replace the globe through shaping a musculoskeletal stump (MS) to improve the position of the prosthesis in the frontal plane and prosthesis mobility, and, consequently, facilitate an improved cosmetic outcome [5, 10]. Not only shaping an MS is required for achieving a cosmetic effect in ocular prosthetics in patients with loss of eye globe. The state and motility of a cosmetic ocular prosthesis are important [11, 12]. We have previously reported on developing a model of ocular prosthesis with permanent attachment to the anterior surface of the MS with the aim to improve the cosmetic effect in ocular prosthetics in patients that underwent evisceration, improve prosthesis motility and prevent low lid ectropion resulting from prosthesis-induced pressure in the lower lid fornix [10]. This prosthesis attachment method enables good prosthesis mobility, but its disadvantage is the requirement for repeat surgical intervention in 4-5 years. Jordan proposed to couple a porous implant to the ocular prosthesis with a titanium peg system. However, this resulted in serious complications (e.g., infection of implant via peg channel) necessitating implant removal in 3.2% of cases [13-15]. In order to avoid these complications, we proposed a method of shaping a hole in an MS in patients who underwent evisceration for ocular trauma or slow uveitis [16]. This method of shaping a hole in an implant and covering the hole with optic nerve sheaths allows avoiding implant exposure during peg invasion in the stump, which was noted by others [13-15]. The purpose of the study was to develop a new technique of evisceration or enucleation secondary to trauma, chronic uveitis or uveal melanoma, with permanent and removable fixation of an ocular prosthesis in a musculoskeletal stump. Material and Methods This retrospective study involved 196 patients (103 men and 93 women) aged 15 to 84 years with chronic uveitis secondary to trauma, phthisis bulbi or uveal melanoma. Group 1 comprised 52 patients (36 men and 16 women) with chronic uveitis secondary to trauma and phthisis bulbi who underwent evisceration for sympathetic ophthalmia prevention and improved cosmesis. A polymer composite implant [17] was placed in the scleral capsule in 18 patients, and an Ekoflon polytetrafluoroethylene (PTFE) implant was placed in the scleral capsule in 34 patients. Group 2 comprised 31 patients (14 men and 17 women) with uveal melanoma who underwent enucleation with a PTFE implant used to shape an MS with a hole in it. Group 3 (the control group for comparison of prosthesis motility with groups 1 and 2) comprised 113 patients (53 men and 60 women). Of these 113 patients, 13 underwent evisceration for chronic uveitis and phthisis bulbi, and 100 underwent enucleation for uveal melanoma, with a PTFE implant used to shape an MS without a hole in it for the pegged prosthesis. Two points essential for the quality of surgical treatment were intentionally taken into account while shaping the MS. First, the potential minimum thickness of future prosthesis equals the difference in prominence between the anterior surfaces of the MS and the healthy eye (5-6 mm), and was taken into account when determining the required size of the implant. Second, there should be firm fixation of the stump to the ocular prosthesis by means of a peg, thus providing for better prosthesis motility. The axial length of the affected eye and the degree of enophthalmos were taken into account when determining the required size of the implant. The required size of the implant was determined by subtracting 5 mm (the minimum prosthesis thickness at the center of the cornea should be of 5 mm or thicker) from the sum of the axial length of the affected eye and the degree of enophthalmos, with the required size of the implant ranging from 16 mm to 18 mm. Firm fixation between the stump and the prosthesis was achieved by shaping a hole in the stump and using a peg on the posterior surface of the prosthesis. The operative technique of shaping the stump with a hole in it [16] in patients after evisceration is described below. The conjunctiva and subconjunctiva are dissected away, the anterior segment (the cornea, iris and ciliary body) is removed, and ocular coats are removed. After the sclera is cut with scissors at the 2, 5, 7 and 10 o’clock positions, optic neurectomy is performed at 6-8 mm from the sclera, and hemostasis is conducted. The posterior pole of the sclera with optic nerve remnants is shifted anteriorly, optic nerve fibers and optic disc are removed, and the distal optic nerve sheath is sutured with gut sutures. After the optic nerve sheaths are placed in the implant hole and fixated to the implant at the bottom of the hole, the scleral shell is turned inside out. A proper size implant with a 4-mm diameter and 5-mm deep hole in the anterior portion of the implant is introduced into the scleral shell and if required additionally fixated to the sclera. Dissolving sutures are placed on the Tenon’s capsule, and silk sutures are placed on the conjunctiva. A polymethyl methacrylate (PMMA) mushroom-like model prosthesis is placed in the conjunctival sac, with its 3-mm-diameter and 5-mm-long peg inserted in the implant hole for 10 days to prevent tissue ingrowth of the hole. A temporary tarsorrhaphy is applied for 8 days, and a tight monocular bandage, for 3-4 days. Matching the peg of the ocular prosthesis with the hole shaped on the anterior surface of the MS is important for achieving the symmetric position of the ocular prosthesis with respect to the healthy eye. This is due to the fact that hole window may shift with respect to the center of the stump after resolution of orbital edema and healing of the wound. Our approach consists in (a) placing a transparent plastic crown inside the conjunctival sac against the hole of the MS, (b) marking the crown with a marker, (c) shaping a peg 5 mm long and 3 mm thick at the posterior surface of the crown, and (d) shaping the rest of the prosthesis [18]. In 3 months, shaping of the conjunctival sac is completed, and a permanent pegged prosthesis is fabricated for the patient. The steps of the surgical procedure are shown in Fig. 1.
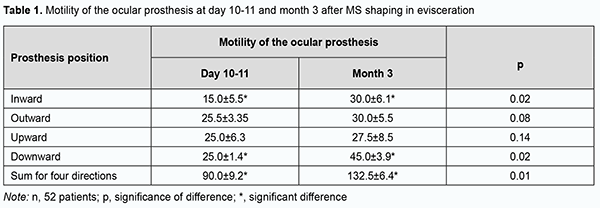
Our operative technique of enucleation and shaping a movable PTFE stump is described below. After an eyelid retractor is placed, 3.0 ml normal saline is injected subconjunctivally. The conjunctiva is incised along the limbus and dissected to the posterior pole of the globe, and the outer rectus muscles are sutured to the sclera and cut away from it. High-frequency electric current is used to dissect the optic nerve and the vascular plexus (ophthalmic artery and superior ophthalmic vein) [19]. After removal of the globe, an 18.0-19.0-mm PTFE implant with a 5x5 mm hole and loop-shaped gut suture No.2 at the hole is placed in a shaped cavity, with the free ends sutured through the wall of the hole to the outer and inner poles of the implant. The outer rectus muscles are secured to the implant at the equator at the 3, 6, 9 and 12 o’clock positions. Thereafter, a silk suture No.6.0 is placed centrally at the conjunctival margin and passed through the gut suture loop located in the hole of the implant. The upper margin of the conjunctiva is sutured and the knot is formed. The free ends of the gut suture are tightened which results in conjunctivalization of the wall of the implant hole. Gut sutures No. 1.5 are placed on the subconjunctiva, and knot sutures (silk sutures No. 6.0), on the conjunctiva. After disinfection eye drops are instilled, a PMMA mushroom-like model prosthesis is placed in the conjunctival sac, with its 3-mm-diameter and 5-mm-long peg inserted in the implant hole for 10 days to prevent tissue ingrowth of the hole. A temporary tarsorrhaphy is applied for 8 days, and a tight monocular bandage, for 3-4 days. The ocular prosthesis is implanted in the conjunctival sac at day 10 or day 11 [20]. In 3 months, shaping of the conjunctival sac is completed, and the permanent pegged prosthesis is fabricated for the patient. Clinical status of the orbital tissues and the results of cosmetic prosthesis were assessed based on the orbital tissue edema (presence or absence of conjunctival chemosis), wound healing (presence or absence of diastasis of the margins of the conjunctival wound), and total mobility of the ocular prosthesis. The functional outcome was assessed by the degree of motility of the ocular prosthesis using the Hirschberg test which reflects the reflex from the anterior surface of the ocular prosthesis with respect to the prosthesis portion simulating the limb of the healthy eye. Data are presented as mean and standard deviation. Results In group 1, a polymer composite implant was placed in the scleral capsule to shape an MS in 18 of 52 patients with chronic uveitis secondary to trauma and phthisis bulbi. In all these patients, moderate orbital edema and no conjunctival chemosis was noted, and a good conjunctival scar developed at day 8, which made it possible to remove sutures and proceed to prosthesis. In addition, no diastasis of wound margins with implant exposure was observed over follow-up. An Ekoflon PTFE implant was placed in the scleral capsule in 34 of 52 patients with chronic uveitis secondary to trauma and phthisis bulbi of group 1, with the postoperative period being not substantially different from that in those group 1 patients who received a polymer composite implant in the scleral capsule to shape an MS. The temporary ocular prosthesis was implanted in the conjunctival sac at day 10 or day 11 for 3 months, and patients were discharged with recommendations and followed by an oculist at their permanent places of residence. It is noteworthy that no complication such as orbital hemorrhage or hematoma, diastasis of the margins of the conjunctival wound, deformity of the conjunctival sac, or implant infection was observed in any patient of group 1. At the 3-month follow-up, a 3-mm diameter and 5-mm deep hole with completely epithelialized walls was seen on the anterior surface of the MS in all patients of group 1. No implant exposure was observed over the follow-up period. Prosthesis motility was assessed at day 10 or day 11 and at month 3 (Table 1).
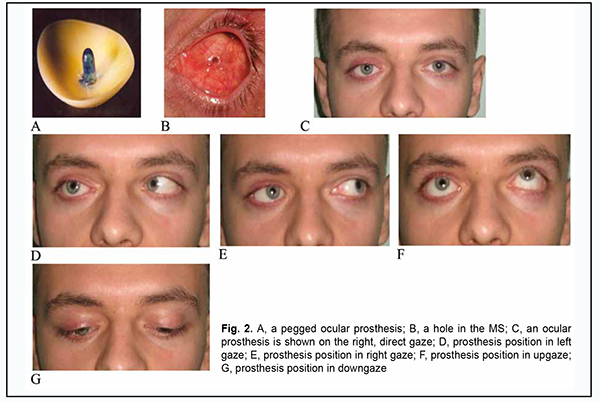
At 3 months after evisceration of the globe and stump shaping, total prosthesis motility improved by 42.5˚ in all directions, and this improvement was statistically significant (p = 0.01). This indicates that it is feasible to perform custom prosthesis 3 months after regression of orbital edema. It is noteworthy that the developed technique of ocular prosthesis after evisceration allowed improving total prosthesis motility at 3 and 12 months to 132.50 ± 6.40 and 147.30 ± 6.70, respectively, versus 103.70 ± 18.30 and 103.10 ± 6.00, respectively, in the controls. Fig. 2 shows a custom pegged ocular prosthesis and its positions 3 months after evisceration of the right eye in a 31-year old patient diagnosed with chronic uveitis secondary to trauma and phthisis bulbi.
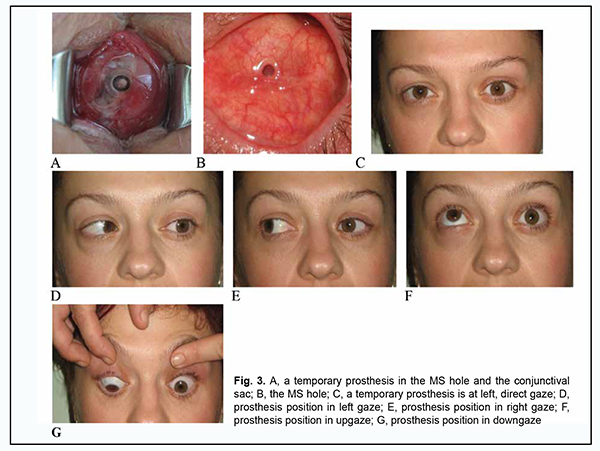
Thirty-one uveal melanoma patients of group 2 exhibited orbital tissue edema and conjunctival chemosis after they underwent enucleation and had a PTFE implant used to shape an MS with a hole in it. In three of these patients, diastasis of the conjunctival margins with implant exposure was observed at the margin of the hole at months 3 and 7, which necessitated implant removal. Total prosthesis motility in the four meridians at 3 and 12 months was 141.60 ± 14.70 and 142.20 ± 16.10, respectively, versus 106.10 ± 13.00 and 103.70 ± 18.30, respectively, in the controls. Fig. 3 shows positions of a custom pegged prosthesis in a 51-year-old female patient at 3 months after left eye enucleation for uveal melanoma.
Therefore, in both study groups with a novel technique used for shaping an MS with a hole in it for a pegged prosthesis, patients had an improvement in the motility of the ocular prosthesis both early and late (3 months and 12 months, respectively) after surgery, which allowed for an improved cosmetic effect. Discussion The state and motility of the ocular prosthesis are essential in cosmetic ocular prosthetics [14, 19]. We have previously reported on developing a model of ocular prosthesis designed to be permanently fixed to the anterior surface of the MS with the aim to improve the cosmetic effect in ocular prosthetics in patients that underwent evisceration, improve prosthesis motility and prevent low lid ectropion resulting from prosthesis-induced pressure in the lower lid fornix [10]. This prosthesis attachment method enables good prosthesis mobility, but its disadvantage is the requirement for repeat surgical intervention in 4-5 years. Jordan proposed to couple a porous implant to the ocular prosthesis with a titanium peg system. However, this resulted in serious complications (e.g., infection of implant via peg channel) necessitating implant removal in 3.2% of cases [8, 11, 13]. Our clinical studies have demonstrated that the proposed method of shaping a hole for the pegged prosthesis at the expense of optic nerve sheaths prevented implant infection [21, 22]. The technique we developed for firm fixation of the ocular prosthesis to the stump after evisceration allowed improving total prosthesis motility in the four meridians at 3 months and 12 months after surgery to 132.50 ± 6.40 and 147.30 ± 6.70, respectively, versus 103.7±18.30 and 103.1 ± 6.00, respectively, in the controls. It is noteworthy that no implant exposure was observed with the implant placed in the scleral capsule after evisceration for chronic inflammation, enabling a conclusion that the scleral coat of the eye prevents an impairment of regeneration processes in the subconjunctiva and conjunctiva. In addition, in most patients, a stable effect in the shaped MS was observed at 12 months and 5 years after surgery, enabling a conclusion that the used implants have no tendency for resorption. An advantage of PTFE implants and polymer composite implants is that their shape can be corrected during surgery. Conclusion We developed methods of shaping a hole in the MS for the pegged ocular prosthesis with the help of either the optic nerve sheaths (after evisceration) or the conjunctiva (after enucleation); these methods can be used for enhancing the motility (and, consequently, the cosmetic effect) of the ocular prosthesis and preventing implant infection and exposure. We found that firm fixation of the pegged ocular prosthesis in the MS allowed improving total prosthesis motility in the four meridians at 3 months and 12 months after evisceration, by 28.8о and 44.2о, respectively, and at 3 months and 12 months after enucleation, by 35.5о and 38.5о, respectively.
References 1.Grusha OV, Lutsevich EA, Grusha IaO. [Major principles of treatment of traumatic orbit deformations]. In: [Proceedings of the symposium on the current problems in ophthalmology]. 26-27 Sep, 2003. Moscow, Russia. P.18-9. Russian. 2.Mosiak NA. [Comparative assessment of different methods of shaping a musculoskeletal stump after enucleation: an experimental and clinical study]. Abstract of Thesis for the Degree of Cand Sc (Med). Filatov Institute of Eye Diseases and Tissue Therapy. Odesa, Ukraine; 1989. Russian. 3.Karcioglu ZA. Actinomyces infection in porous polyethylene orbital implant. Graefe's Arch Clin Exp Ophthalmol. 1997 Jul; 235(7): 448-51. 4.Anina IeI, Levtiukh VI. [Surgical and therapeutic restoration of vision]. In: [Proceedings of the twelfth symposium in ophthalmology]. June 29 – July 1, 2001, Chernivtsi, Ukraine. p.8. Ukrainian. 5.Vasil'eva SF, Gorgiladze TU, Grachev NN. [Method of enucleation with the formation of a mobile base for the prosthesis]. Oftalmol Zh. 1986;(1):61-2. Russian. 6.Krasilnikova VL, Kovalenko IuD, Iakhnitskaia LK. [Delayed muscoskeletal stump plasty with a composite implant based on aluminum oxide ceramic foam and nanocrystal hydroxyapatite]. In: [Proceedings of the 2nd Congress of the Black Sea Ophthalmological Society]. 8-10 Sep, 2004. Odesa, Ukraine. p.145. Russian. 7.Bigun NM. [New surgical approaches and implant materials in reconstructive surgery in the orbital and periorbital area: experimental and clinical studies]. Thesis for the Degree of Cand Sc (Med). Filatov Institute of Eye Diseases and Tissue Therapy. Odesa, Ukraine; 2019. Ukrainian. 8.Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report no. 1. Arch Ophthalmol. 1990 Sep;108(9):1268-73. 9.Gundorova RA, Khoroshilova-Maslova IP, Bykov VP, et al. [Experimental and morphological results of implantation of carbon felt in plastic surgery of the locomotor stump and accessory system of the eye]. Vestnik Oftalmologii. 1996; 1: 27-31. Russian. 10.Maletskyi AP, Samchenko IuM, Vit VV, Bigun NM, Kernosenko LO. [Response of orbital and auricular soft tissues to the developed hydrogel implant in rabbits]. Arkhiv oftalmologii Ukrainy. 2018;6(2):20-7. Ukrainian. 11.Simchuk IV. [Analysis of results of surgical treatment and ocular prosthesis after evisceronucleation]. Arkhiv oftalmologii Ukrainy. 2016;4(1):72-80. Ukrainian. 12.Kaltreider SA, Newman SA. Prevention and management of complications associated with the hydroxyapatite implant. Ophthalmic Plast Reconstr Surg. 1996 Mar; 12 (1):18-31. 13.Hopper RH Jr, Engh CA, Fowlkes LB, Engh CA. The pros and cons of polyethylene sterilization with gamma irradiation. Clin Orthop Relat Res. 2004 Dec;(429):54-62. 14.Jordan D R, Klapper SR. Wrapping hydroxyapatite implants. Ophthalmic Surg Lasers. 1999; 30 (5): 403-407. 15.Jordan DR, Gilberg S, Bawazeer A. Coralline hydroxyapatite orbital implant (bio-eye): experience with 158 patients. Ophthalmic Plast Reconstr Surg. 2004 Jan;20(1):69-74. 16.Maletskyi AP. [A method of shaping a hole in the musculoskeletal stump in patients after evisceration secondary to trauma and slow uveitis]. Patent of Ukraine № 46980 A61F 2/14. Information Bulletin No.1 issued Jan 11, 2010. Ukrainian. 17.Dubkova VI, Glinnik AV, Maletskyi AP, Maievskaia OI, Bigun NM. [Carbon-fiber composite material for soft tissue defect repair]. Patent of Belarus № 121881 issued Jan 29, 2018. Russian. 18.Bigun NM, Maletskyi AP, Melnichenko LM, Makarova IV, Demidova LV. [A method of comparing the peg of the ocular prosthesis with a hole in the musculoskeletal stump in patients after evisceration or enucleation secondary to trauma, slow uveitis or intraocular neoplasm]. Patent of Ukraine № 135224 A 61F 2/00. Information Bulletin No.12 issued Jun 25, 2019. Ukrainian. 19.Pasyechnikova NV, Maletskyi AP, Naumenko VO, Poliakova SI, Chebotariov EP, Pukhlik ES. [Use of high-frequency electric welding in enucleation for uveal melanoma]. In: [Proceedings of the 12th Congress of Ukrainian ophthalmologists]. 26-28 May, 2010. Odesa, Ukraine. p.22. Russian. 20.Maletskyi AP. [A method of shaping a hole in the musculoskeletal stump in patients after enucleation]. Patent of Ukraine № 46980 A61F 2/14. Information Bulletin No.1 issued Jan 11, 2010. Ukrainian. 21.Maletskyi AP, Bushuieva NM, Chebotariov EP. [Ocular prosthesis]. Patent of Ukraine № 11682 A61F 2/14. Information Bulletin No.1 issued Jan 16, 2006. Ukrainian. 22.Maletskyi AP, Dubkova VI, Maievska OI, Bigun NM. [Application of polymer composite materials in shaping a musculoskeletal stump after evisceration: Preliminary results]. Oftalmol Zh. 2013;(6):57-61. Russian.
Disclosures Corresponding Author: Maletskyi A. P., Email: maletskiy@filatov.com.ua Author Contributions: Maletskyy AP: Conceptualization, Project administration, Data Curation, Writing – original draft, Writing – review & editing Bigun NM: Data Curation, Writing – original draft, Writing – review & editing All authors reviewed the results and approved the final version of the manuscript. Funding sources: The study is a portion of the Development of Novel Synthetic Polymer Implants with Immobilized Biologically Active Substances and Assessment of Tissue Response at the Orbital and Periorbital Area, a research program. Conflict of interest: The authors state that they have no conflict of interest that might bias this work.
The study was conducted in the clinical setting. Ethical standards were adhered to throughout the study
|