J.ophthalmol.(Ukraine).2022;5:12-18.
|
http://doi.org/10.31288/oftalmolzh202251218 Received: 03.08.2022; Accepted: 22.08.2022; Published on-line: 27.10.2022 Clinical manifestations of keratitis and corneal ulcers in patients with rheumatoid arthritis: a retrospective analysis L. Y. Riazanova, G. I. Drozhzhyna SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE: Riazanova LY, Drozhzhyna GI. Clinical manifestations of keratitis and corneal ulcers in patients with rheumatoid arthritis: a retrospective analysis. J.ophthalmol.(Ukraine).2022;5:12-8. http://doi.org/10.31288/oftalmolzh202251218 Background: The prevalence of keratitis and corneal ulcers among rheumatoid arthritis (RA) patients with corneal disease is not known for certain, and the features of their clinical manifestations have been not sufficiently studied. Purpose: To retrospectively assess the prevalence and clinical manifestations of keratitis and corneal ulcers in RA patients with corneal disease based on the medical records of patients that were hospitalized at Corneal Pathology Department of the Filatov Institute from January, 2014, through August, 2019. Material and Methods: We have retrospectively examined the medical records of 6627 patients that were hospitalized at Corneal Pathology Department of the institute in the above period of time. Results: Of the 6627 patients that were hospitalized, 82 (or 1.2%) were RA patients with keratitis and/or corneal ulcers. Of these 82 patients aged 37 to 79 years, 23 (28%) were men and 59 (72%) were women. Bilateral corneal lesions were found in 71 (86.6%), and unilateral corneal lesions, in 11 (13.4%) of the 82 study patients, with 153 eyes totally included in the study. Punctate or filamentary keratitis was found in 90 (58.8%) eyes. Severe corneal lesions (ulcers or keratoscleromalacia) were found in 63 (41.2%) eyes. Of the 82 RA patients with corneal lesions of the current study, 39 (47.6%) did not receive basic therapy for RA, and exhibited the most severe corneal lesions. Conclusion: Patients with RA need to be systematically seen by an ophthalmologist and a rheumatologist. The absence of treatment with basic therapy for RA can cause ocular complications. A patient with RA needs to be treated for any corneal lesion at a tertiary care center where a required surgical procedure can be timely performed. The success of treatment for corneal lesions in a RA patient requires a set of treatment measures, and adequate basic therapy for RA is a necessary component of this set. Keywords: keratitis, corneal ulcer, keratoscleromalacia, rheumatoid arthritis
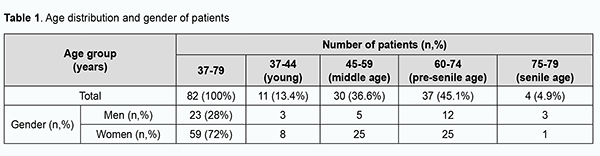
Introduction Rheumatoid diseases are one of the most common pathologies globally, particularly in Ukraine. Among them, the most common is rheumatoid arthritis (RA), a systemic autoimmune disease that affects joints and extra-articular organs such as lungs, pericardium, skin and ocular tissues. According to WHO data, RA affects 0.6-1.5% of people. The prevalence of RA in Ukraine is 0.4%, and in Europe and Northern America, 1-2% [1, 2, 3]. The disease is two to four times more common in women than in men. The overall number of patients with RA in Ukraine is about 125,000 [4]. The disease affects individuals of different age groups and has a progressive course, resulting in loss of capacity to work and early disability [5], and is characterized by the possibility of extra-articular lesions [6]. Autoimmune diseases may have ocular involvement, but more research is required to understand the epidemiology of these manifestations [7]. In a study by Bettero and colleagues [8], Sjögren's syndrome was the most common ocular manifestation, whereas ulcerative keratitis and scleritis appeared in 2% of RA patients each. Daguano and colleagues [9] reported that the main ocular manifestation of RA was dry eyes (secondary Sjögren's syndrome), followed by scleritis, peripheral ulcerative keratitis and uveitis. Corneal inflammation is more common in female patients, whereas retinal vasculitis is more common in male patients with RA [10]. In a study by Syniachenko and colleagues [11], an ocular disease was found in a fifth of patients with RA, and the predominant type of ocular manifestation in RA was uveitis, followed by scleritis, keratitis, glaucoma, cataract and conjunctivitis, with the approximate ratio of 10 : 6 : 5 : 4 : 4 : 1. Others have reported the prevalence of keratitis, scleritis and uveitis in patients with RA to range from 12 to 30% [12, 13]. Numerous theories exist to explain the etiopathogenesis of RA [14, 15, 16, 17, 18], but it has not been fully elucidated. Most researchers support the immunologic theory based on the finding of rheumatoid factor (RF; antibodies directed against the Fc region of IgG) in individuals with RA. It is likely that CD4 T cells, mononuclear phagocytes, fibroblasts, osteoclasts and neutrophils play major cellular roles in the pathophysiology of RA, while B lymphocytes produce autoantibodies (ie, RF). RF may be present in other conditions, and in some healthy people, and cannot be a pathognomonic sign of RA. A persistently high RF titer in an asymptomatic individual may indicate an increased risk of developing RA [14]. Another major theory is a genetic theory. RA has a significant genetic component and shared epitope of HLA-DR4/DR1 cluster is present in up to 90% of patients with RA. Genetic factors and immune system abnormalities contribute to disease propagation. Inflammation and exuberant proliferation of synovium (ie, pannus) leads to destruction of various tissues, including cartilage, bone, tendons, ligaments, and blood vessels. Although the articular structures are the primary sites involved by RA, other tissues are also affected. Extra-articular involvement of organs such as the skin, heart, lungs, and eyes can be significant [14, 19, 20, 21] and is seen in 10% to 20% of patients, mostly seropositive [14]. It is important that, in 25-30% of patients with RA, ocular lesions develop already in early disease [22]. Over the recent years, there has been an increase in the number of RA patients presenting with keratitis and corneal ulcers to Corneal Pathology Department of the Filatov Institute. However, the prevalence of keratitis and corneal ulcers among RA patients with corneal disease is not known for certain, and their clinical manifestations have been not sufficiently studied. The purpose of the study was to retrospectively assess the prevalence and clinical manifestations of keratitis and corneal ulcers in RA patients with corneal disease based on the medical records of patients with RA presenting to Corneal Pathology Department of the Filatov Institute from January, 2014, through August, 2019. Material and Methods We have retrospectively examined the medical records of 6627 patients that were hospitalized at Corneal Pathology Department of the Filatov Institute from January, 2014, through August, 2019. A research data base was developed during this study. The objective description of patient state was based on the analysis of demographic characteristics as per the WHO age group classification and history data regarding basic therapy for RA, erythrocyte sedimentation rate (ESR), leukocyte count (LC), RF level and C-reactive protein (CRP). Ophthalmological data were analyzed with respect to clinical diagnosis, corneal defect location and size, microbiological findings of conjunctival discharge, corneal status at admission and at discharge (epithelialization of the corneal surface as assessed by fluorescein staining), visual acuity, corneal and limbal vascularization as assessed by biomicroscopy, and intraocular pressure (IOP) at admission and at discharge. In patients with RA, corneal lesions were divided as per clinical diagnosis into punctate or filamentary keratitis, non-perforated corneal ulcer, perforated corneal ulcer, and keratoscleromalacia. In addition, corneal lesions were divided by location into central and peripheral lesions. Fluorescein staining test results were categorized as negative (no staining), epitheliopathy (isolated or diffuse punctate epithelial erosion), corneal stromal defect < 4 mm, or corneal stromal defect ≥ 4 mm. Vascularization severity was categorized as no; 1 vascularized quadrant; 2 vascularized quadrants; 3 or 4 vascularized quadrants; or vascularization of the limbus only. The IOP was assessed by palpation in all cases due to the status of the cornea and categorized as normal, compensated by topical drugs, hypertension or hypotony. During treatment, patients received topical therapy including antiseptics (4-6 times a day), antibiotics (fluoroquinolones or aminoglycosides, 4-6 times a day), proteolytic enzyme inhibitors (intravenously, 6 times a day), mydriatics (1-2 times a day), Dexpanthenol gel (4 times a day), tear substitutes (containing hyaluronic acid 0.12-0.4%, 6 times a day), and hypotensive agents (beta blockers, carboanhydrase inhibitors or their fixed combinations, twice a day). The mean duration of conservative therapy was 18 ± 3.7 days. Basic therapy for RA included cytostatics (methotrexate), aminoquinolones (delagil), corticosteroids (methypred or prednisolone) in doses recommended by a rheumatologist. Analysis of variance and descriptive statistics were performed using Statistica 12 software. The McNemar test was used to test for statistically significant differences. The level of significance p ≤ 0.05 was assumed. Results Of the 6627 patients that have been hospitalized at Corneal Pathology Department of the Filatov Institute, 82 (or 1.2%) were RA patients with keratitis and/or corneal ulcers, and were retrospectively included in the study. Of these 82 patients aged 37 to 79 years, 23 (28%) were men and 59 (72%) were women, with a male to female ratio of 1:2.6. The mean patient age was 59.1 ±10.3 years for the study group, 60.6 ± 9.8 years for men and 58.5 ±10.5 years for women, with no significant difference between men and women. Of the 82 patients, 67 (81.7%), the vast majority, were RA patients aged 45 to 74 years (Table 1).
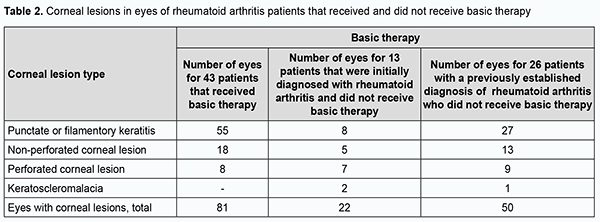
In addition, of the 82 patients, 69 (84.1%) had known their diagnosis of RA. Of the 69 patients, 43 had been receiving and 26 had not been receiving basic therapy for RA. Moreover, of the 82 patients, 13 (15.9%) were initially diagnosed with RA by a rheumatologist during their inpatient stay at Corneal Pathology Department. A normal ESR was seen in 45 of the 82 patients. An elevated ESR was seen in 37 patients (45.1%), including 13 men (15.8%) with an ESR of 12-43 mm/year, and 24 women (29.3%) with an ESR of 16-55 mm/year. Only 4 patients had a leukocyte count of 9.2 to 14 × 109/L, i.e., higher than a norm of 4-9×109/L. Of the 82 patients, only 24 (29.3%) had their serum RF levels measured, and, in all these cases, the serum RF levels were higher than a norm of ≤ 14 IU/mL, ranging from 22.13 IU/mL to 359.8 IU/mL. Of the 24 patients (29.3%) having their C-reactive protein measured, 10 (12.2%) had a normal C-reactive protein level of ≤ 5 mg/L, and 14 (17%) had an increased C-reactive protein level ranging from 7.71 до 255.85 mg/L. Bilateral corneal lesions were found in 71 (86.6%), and unilateral corneal lesions, in 11 (13.4%) of the 82 study patients, with 153 eyes totally included in the study. Severe corneal lesions (ulcers or keratoscleromalacia) were found in 63 (41.2%) eyes, including 36 (23.5%) with non-perforated corneal ulcers, 24 (15.7%) with perforated corneal ulcers, and 3 (2%) with keratoscleromalacia. Punctate or filamentary keratitis was found in 90 (58.8%) eyes, and was rather common both in eyes receiving and in eyes not receiving (55 eyes and 35 eyes, respectively) basic therapy for RA. Non-perforated corneal ulcers were seen in 18 eyes receiving and in 18 eyes not receiving basic therapy for RA. Perforated corneal ulcers were twice more common in eyes not receiving than in eyes not receiving basic therapy for RA (16 eyes versus 8 eyes, respectively). Keratoscleromalacia was seen only in eyes not receiving basic therapy for RA. Therefore, the most severe corneal lesions (i.e., perforated corneal ulcers and keratoscleromalacia) were more common in eyes not receiving basic therapy for RA (Table 2).
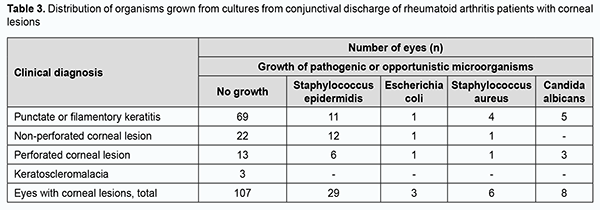
Central corneal ulcers and peripheral corneal ulcers were seen in 25 (16.3%) eyes and 30 (19.6%) eyes, respectively. Ulcers were large and involving both central and peripheral cornea in 5 (3.3%) eyes. Corneal lesions were peripheral in eyes with keratoscleromalacia. Pathogenic microflora (like Escherichia coli and/or Staphylococcus aureus) was found in the conjunctival discharge of 9 (5.9%) eyes, and opportunistic pathogens (like Staphylococcus epidermidis and/or Candida albicans), in the conjunctival discharge of 37 (24.2%) eyes of the 153 eyes included in the study (Table 3).
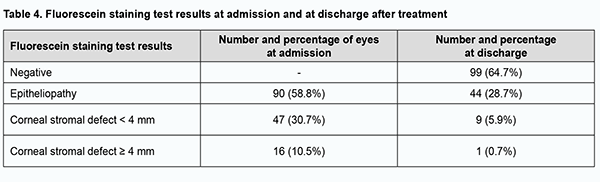
Of the 153 eyes, 108 (70.6%) required medical therapy only, 19 (12.4%), medical therapy and soft contact lens, and 26 (17%), surgical treatment for corneal lesions during patients’ inpatient stay at Corneal Pathology Department. The multiagent medical therapy involved topical antiseptics, proteolytic enzyme inhibitors, repair agents, antibiotics and hypotensive agents (when indicated), glucocorticosteroids, and preservative-free tears substitutes. The basic systemic therapy (methotrexate and glucocorticosteroids) for RA was administered, if prescribed by a rheumatologist. Surgical treatment involved lamellar keratoplasty in 9 eyes, penetrating keratoplasty in 3 eyes, step-by-step penetrating keratoplasty in 3 eyes, amniotic membrane transplantation in 1 eye, blepharorrhaphia in 2 eyes, lamellar xenokeratoplasty in 1 eye, and surgical opening of the palpebral tissue in 3 eyes. A combination surgical procedure (lamellar keratoplasty with blepharorrhaphia, amniotic membrane transplantation with blepharorrhaphia, or penetrating keratoplasty with amniotic membrane transplantation) was performed in 3 eyes. Biological dressing by Puchkovska (n = 1), blepharorrhaphia (n = 1; due to lamellar corneal graft lysis), or phacoemulsification of the complicated cataract (n = 1) was performed as the second stage of surgery in 4 eyes. Fluorescein staining was positive at admission in all study eyes. After treatment, corneal epithelialization was complete in 99 (64.7%) eyes (р = 0.00), epitheliopathy was still present in 44 (28.7%) eyes, and stromal defects, in 10 (6.6%) eyes (Table 4).
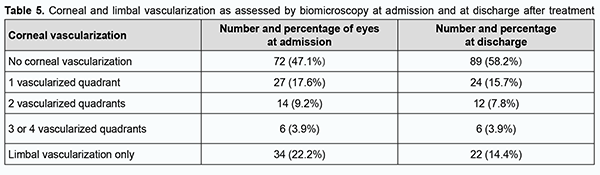
No corneal vascularization was seen in 72 (47.1%) eyes at admission and in 89 (58.2%) eyes after treatment, i.e., vascular regression was observed in 17 (11.1%) eyes (Table 5).
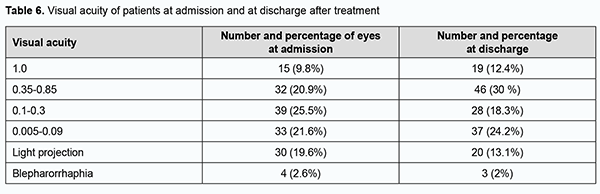
The difference between the percentages before and after treatment was significant by McNemar's χ2 test (χ2 = 15.6; р = 0.0001). At admission, ocular hypertension was seen in 9 (5.9%) eyes, hypotony, in 24 (15.7%) eyes, IOP compensated by topical drugs, in 14 (9.1%) eyes, and normal IOP, in 106 (69.3%) eyes. At discharge, normal IOP was seen in 123 (80.4%) eyes, ocular hypertension, in 2 (1.3%) eyes, hypotony, in 1 (0.7%) eye, and IOP compensated by topical drugs, in 27 (17.6%) eyes. After treatment, the IOP normalized in 17 (11.1%) eyes, and improved to a compensated state, in 13 (8.5%) eyes. At admission, visual acuity was light projection in 30 (19.6%) eyes, ranged from 0.005 to 0.09 in 33 (21.6%) eyes, from 0.1 to 0.3 in 39 (25.5%) eyes, from 0.35 to 0.85 in 32 (20.9%) eyes, and 1.0 in 15 (9.8%) eyes, and not measurable due to blepharorrhaphy in 4 (2.6%) eyes. After in-patient treatment, visual acuity was light projection in 20 (13.1%) eyes, ranged from 0.005 to 0.09 in 37 (24.1%) eyes, from 0.1 to 0.3 in 28 (18.3%) eyes, from 0.35 to 0.85 in 46 (30%) eyes, and 1.0 in 19 (12.4%) eyes, and not measurable due to blepharorrhaphy in 3 (2%) eyes. There was a significant increase (p = 0.01) in the number of eyes with high visual acuity (i.e., visual acuity of 0.35 to 1.0) from 47 (30.7%) at admission to 65 (42.5%) after treatment, indicating a positive treatment outcome and improvement in patients’ quality of life (Table 6).
Discussion Management of RA is a medical and social challenge due to a progressive course, low efficacy of treatment, high prevalence of the disease, and high rate of loss of capacity to work, with half or more patients losing their capacity to work at 3-5 years after disease onset [23]. Our retrospective analysis found that severe corneal lesions (ulcers or keratoscleromalacia) were seen in 63 (41.2%) eyes, presenting a potential threat of losing vision and the eye. It was believed that RA is severe medical condition treated with corticosteroids for a long time, which frequently resulted in corneal lesions in the form of pure ulcers [24]. Others have taken another point of view on the pathogenesis of peripheral corneal lesions in autoimmune disorders, believing that immune complex deposition may trigger a local immune response, leading to migration of immunocompetent cells from perilimbal vessels and activation of complement components. Neutrophils infiltrate the peripheral cornea and release proinflammatory factors, causing degradation of the corneal stroma [25, 26, 27]. The latter point of view is still alive [28]. It is known that a persistent ulcerative corneal defect can be complicated by purulent infection. Destruction of the corneal stroma results in denudation of Descemet’s membrane and impending perforation, which can lead to the loss of the eye [29]. Cases of corneal ulcers associated with RA and complicated by pathogenic microorganisms have been reported. Singh and colleagues [30] believe that the onset of corneal melting in a case of seropositive rheumatoid arthritis associated with corneal melting in the absence of other typical clinical manifestations of rheumatoid arthritis flare could represent either RA progression or development of severe eye infection while on immunosuppressive regimen. In the opinion of Ide and colleagues, immunosuppressive therapy is a major risk factor of corneal bacterial infections in RA-associated corneal ulceration, although it has been previously believed that RA-associated peripheral ulcerative keratitis should be managed with aggressive immunosuppression if the associated morbidity and mortality are to be avoided [32, 33]. In the current study, pathogenic and opportunistic microorganisms were detected in 9 (5.9%) eyes and 37 (24.2%) eyes, respectively, and no growth of pathogenic or opportunistic microorganisms was seen in 107 (69.9%) eyes, which could indicate a prevailing involvement of not an infectious but an autoimmune component in the development of severe corneal lesions in our patients with RA. An elevated ESR was seen in 37 (45.1%) patients, and an elevated leukocyte count, in only 4 (4.9%) patients. An elevated serum RF level was, however, seen in all the 24 (29.3%) patients that had their serum RF level measured; this could indicate a direct role of RF in the development of corneal lesions in patients with RA. It is interesting that in a study by Syniachenko and colleagues [11], there was no substantial difference in serum RF levels between RA patients with no eye disease and RA patients with eye disease, but seropositive RA was significantly and 63% more frequently seen in the former patients. Itty and colleagues [34] demonstrated that RA patients who were both anti-anti-cyclic citrullinated peptide and RF positive tended to have more and worse ocular disease. In the current study, of the eyes with corneal lesions, 81% received conservative therapy (including those that were dressed with a therapeutic soft contact lens), and 19% required surgical treatment, with 36 surgical procedures performed, including lamellar keratoplasty, lamellar-and-penetrating keratoplasty, penetrating keratoplasty, amniotic membrane transplantation, blepharorrhaphia, lamellar xenokeratoplasty and combinatory surgical procedures. Solomon and colleagues [29] noted that amniotic membrane transplantation facilitates epithelialization and reduces inflammatory and immune responses and vascularization, which enables achieving a curative effect in patients with deep corneal ulcers in the presence of systemic disorders. A beneficial effect of amniotic membrane transplantation in combination with temporary blepharorrhaphia has been reported. In patients with RA, keratoplasty can be performed in the presence of substantial corneal destruction in order to save the eye, but a repeat keratoplasty may be required for this purpose [35]. Our retrospective analysis of ophthalmological changes showed that the treatment was beneficial in RA patients with keratitis and corneal ulcers. The management of eye disease in patients with rheumatoid arthritis is a medical challenge that requires close cooperation between rheumatologists and ophthalmologists. Of the 82 RA patients with corneal lesions of the current study, only 43 (52.4%) patients received basic therapy for RA, and the most severe corneal lesions (i.e., perforated corneal ulcers and keratoscleromalacia) were more common in eyes not receiving basic therapy for RA. It is believed that early diagnosis of rheumatoid arthritis is essential for prescribing adequate treatment capable of preventing sight-threatening complications. In addition, early identification of ocular manifestations of a systemic rheumatic disease can prevent or delay late complications [34, 36]. Consequently, patients with RA need to be systematically seen by an ophthalmologist, and to be treated for any corneal lesion at a tertiary care center where keratoplasty can be timely performed by a qualified eye surgeon. Clinical observations suggest that severe compli¬cations, such as corneal “melts” and perforations in RA, are decreasing with advances in modern biologic treatments [37]. Conclusion First, Corneal Pathology Department hospitalizations of RA patients with keratitis and/or corneal ulcers were analyzed in relation to total Corneal Pathology Department hospitalizations from January, 2014, through August, 2019. The prevalence of hospitalization of RA patients with keratitis and/or corneal ulcers was 1.2%. Second, bilateral corneal lesions were found in 71 (86.6%). Severe corneal lesions (ulcers or keratoscleromalacia) were found in 63 (41.2%) eyes, including 24 (15.7%) with perforated corneal ulcers. Finally, of the 82 RA patients with corneal lesions of the current study, 39 (47.6%) did not receive basic therapy for RA, which could be a contributor to ocular complications. The success of treatment for corneal lesions in a RA patient requires a set of treatment measures, and adequate basic therapy for RA is a necessary component of this set.
References 1.Kovalenko VM, Kornatskyi VM, editors. [Demographic and health state characteristics of Ukrainian people: an analytical and statistical manual]. Kyiv; 2010. Ukrainian. 2.Majitha V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med. 2007 Nov;120(11):936-9. 3.Mazurov VI, Trofimov EA. [Innovative methods of treatment of autoimmune disorders]. Vestnik Rossiiskoi akademii meditsinskikh nauk. 2015;2:165-8. Russian. 4.Iaremenko OB, Mykytenko AM. [Early diagnosis of rheumatoid arthritis]. Zdorov’ia Ukrainy. 2008;5(1):63-5. Ukrainian. 5.Kovalenko VM, Bortkevych OP, Biliavska IuV. [Current aspects of the diagnosis of rheumatoid arthritis]. Zdorov’ia Ukrainy. 2010;1:74-7. Ukrainian. 6.Neiko IeM, Iatsyshin RI, Shefiuk OV. [Rheumatoid arthritis: a current view on the problem]. Ukrainskyi revmatologichnyi zhurnal. 2009;2(36):35–9. Ukrainian. 7.Levitt A, McManus K, McClellan A, et al. Ocular inflammation in the setting of concomitant systemic autoimmune conditions in an older male population. Cornea. 2015;34:762–7. 8.Bettero RG, Cebrian RF, Skare TL. [Prevalence of ocular manifestation in 198 patients with rheumatoid arthritis: a retrospective study]. Arq Bras Oftalmol. 2008 May-Jun;71(3):365-9. Portuguese. 9.Daguano CR, Bochnia CR, Gehlen M. [Anterior uveitis in the absence of scleritis in a patient with rheumatoid arthritis: case report]. Arq Bras Oftalmol. 2011;74(2):132–3. Portuguese. 10.Hennessy AL, Katz J, Covert D, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011 Dec;152(6):982-8. 11.Syniachenko OV. [Rheumatoid arthritis and ophthalmopathy]. Ukrainskyi revmatologichnyi zhurnal. 2012;50(4):1–5. Russian. 12.Katargina LA, Khvatova AV, Denisova EV. [The effectiveness of trabeculectomy with the use of cytotoxic drugs in the treatment of uveal glaucoma]. Oftalmokhirurgiia. 2002;3:37–40. Russian. 13.Rudakova AV. [Pharmacoeconomic aspects of treatment with the inhibitors of tumor necrosis factor of the chronic uveitis refractory to the basic therapy (including an associated with juvenile idiopathic arthritis)]. Pediatricheskaia farmakologiia. 2011;8(4):55–8. Russian. 14.Sahatçiu-Meka V, Rexhepi S, Manxhuka-Kerliu S, Rexhepi M. Extra-articular manifestation of seronegative and seropositive rheumathoid arthritis. Bosn J Basic Med Sci. 2010 Feb; 10(1): 26–31. 15.Lilleby V, Gran JT. [Systemic rheumatoid arthritis]. Tidsskr Nor Laegeforen. 1997 Nov 30;117(29):4223-5. Norwegian. 16.Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol. 2007;125(7):895–900. 17.Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with rheumatoid arthritis and with other systemic immune-mediated diseases. Ophthalmology. 1994 Jul;101(7):1281-6; discussion 1287-8.. 18.Fong LP, Sainz de la Maza M, Rice BA, Kupferman AE, Foster CS. Immunopathology of scleritis. Ophthalmology. 1991 Apr;98(4):472-9. 19.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–735. 20.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–727. 21.Cimmino MA, Salvarani C, Macchioni P, et al. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19(6):213–217. 22.Zlatanović G, Veselinović D, Cekić S, Zivković M, Dorđević-Jocić J, Zlatanović M. Ocular manifestation of rheumatoid arthritis-different forms and frequency. Bosn J Basic Med Sci. 2010;10(4):323-327. 23.Kovalenko VM, Shuba MM, Sholokhova LB. [Rheumatoid arthritis. Diagnosis and treatment]. Morion; 2001. Ukrainian. 24.Tarasova LN, Kudriashova IuI. [Clinical picture of pure corneal ulcers of different localizations]. Vestn Oftalmol. 1999 Jan-Feb;115(1):29-31. Russian. 25.Gregory JK, Foster CS. Peripheral ulcerative keratitis in the collagen vascular diseases. Int Ophthalmol Clin. 1996 Winter;36 (1): 21-30. 26.Smith VA, Hoh HB. Role of ocular matrix metalloproteinases in Peripheral Ulcerative Keratitis. Br J Ophthalmol. 1999 Dec; 83: 1376-1383. 27.Messmer EM, Foster CS. Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol. 1999; 43 (5): 379-96. 28.Drozdova EA, Timoshevskaia EI. [Differential diagnosis and tactics of treatment of peripheral corneal lesions]. Tochka zrenia. Vostok-Zapad. 2017;1:57–60. Russian. 29.Solomon A, Meller D, Prabhasawat P, et al. Amniotic membrane grafts for nontraumatic corneal perforations, descemetoceles, and deep ulcers. Ophthalmology. 2002 Apr;109(4):694-703. 30.Singh G, Salvador VB, Bagchi A, Tushabe R, Abrudescu A. Rheumatoid arthritis-associated corneal ulceration with superimposed infection by methicillin-resistant Staphylococcus aureus: a complicated type of corneal melt. Am J Case Rep. 2014 Nov 27;15:523-5. 31.Ide T, Matsuda H, Nishida K, et al. Rheumatoid arthritis-associated corneal ulceration complicated by bacterial infection. Mod Rheumatol. 2005;15:454–58. 32.Squirell DM, Winfield J, Amos RS. Peripheral ulcerative keratitis corneal melt and rheumatoid arthritis: a case series. Rheumatology (Oxford). 1999;38(12):1245–48. 33.Perez VL, Azar DT, Foster CS. Sterile corneal melting and necrotizing scleritis after cataract surgery in patients with rheumatoid arthritis and collagen vascular disease. Semin Ophthalmol. 2002;17(3–4):124–30. 34.Itty S, Pulido JS, Bakri SJ, et al. Anti-cyclic citrullinated peptide, rheumatoid factor, and ocular symptoms typical of Rheumatoid arthritis. Trans Am Ophthalmol Soc. 2008;106:75–83. 35.Stepanov VK, Muriieva IV, Isaieva OV. [Curative keratoplasty in destructive corneal disease in patients with rheumatoid arthritis]. Vestnik OGU. 2015;12(187):231-3. Russian. 36.Kemeny-Beke A, Szodoray P. Ocular manifestations of rheumatic diseases. Int Ophthalmol. 2020 Feb;40(2):503-510. 37.Turk MA, Hayworth JL, Nevskaya T, Pope JE. Ocular Manifestations in Rheumatoid Arthritis, Connective Tissue Disease, and Vasculitis: A Systematic Review and Metaanalysis. J Rheumatol. 2021 Jan 1;48(1):25-34.
Disclosures Corresponding Author: Riazanova LY, e-mail: lili.r@ukr.net Athour Contribution: Riazanova L Y: Conceptualization; Data Curation; Formal Analysis; Writing – original draft; Writing – review & editing. Drozhzhyna GI: Conceptualization; Formal Analysis; Project administration;Writing – review & editing. All authors approved the final version of the manuscript and were responsible for submitting it for publication. Disclaimer: The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions. Funding sources: No funding received The study is a portion of Features of the Pathogenesis of Corneal Degenerative (Keratoconus), Inflammatory (Bacterial and Fungal Keratitis) and Autoimmune (Rheumatoid Arthritis, Stevens-Johnson Syndrome and Lyell's Syndrome) Disorders and Developing Novel Methods for Treating Them, a research program (Ukrainian State Registration No. 0119U103094). Conflict of interest: The authors state that they have no conflict of interest that might bias this work. Subjects: The study involved human subjects, was approved by the Ethics Committee, and adhered to the tenets of the Declaration of Helsinki. Informed consent was not obtained due to the retrospective nature of the study.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IOP, intraocular pressure; RA, rheumatoid arthritis; WHO, World Health Organization
|