J.ophthalmol.(Ukraine).2022;4:58-61.
|
http://doi.org/10.31288/oftalmolzh202245861 Received: 07.02.2022; Accepted: 24.03.2022; Published on-line: 24.08.2022 Ocular motor manifestations of neurodegenerative diseases: case reports N. M. Moyseyenko Ivano-Frankivsk National Medical University; Ivano-Frankivsk Ukraine TO CITE THIS ARTICLE: Moyseyenko NM. Ocular motor manifestations of neurodegenerative diseases: case reports. J.ophthalmol.(Ukraine).2022;4:58-61. http://doi.org/10.31288/oftalmolzh202245861
Background: Ocular motor manifestations (nystagmus and diplopia) do not lead to blindness. However, loss of cognitive abilities in the presence of these manifestations can lead to irreparable social consequences. Therefore, improved diagnostic assessment of ocular motor abnormalities will enable early detection of neurodegenerative diseases, whereas the correction of these abnormalities will prevent the undesirable consequences. Purpose: To analyze the nature of ocular motor manifestations of neurodegenerative disorders. Material and Methods: Two cases are presented: a case of a partial Parinaud's syndrome, and a case of viral encephalomyelitis. Results: In a case of a partial Parinaud's syndrome, an examination found ocular motor manifestations in the form of incongruous diplopia, nystagmus at near and pupillary light-near dissociation. This allowed suspecting pons lesions, which was confirmed by MRI evidence of multiple small degenerations at the dorsal-medial pons, the location specific for a Parinaud's syndrome. In case 2, the patient was diagnosed with viral encephalomyelitis and substantial bilateral vertical rotational nystagmus, more pronounced in abduction, indicating damage to the brainstem. Conclusion: The pattern and nature of ocular motor abnormalities are helpful in localizing lesions of such deep brain structures as the brainstem and pons. A more thorough study will be beneficial for early diagnostic assessment and timely treatment of neurodegenerative lesions, and, consequently, for preventing undesirable social consequences. Keywords: ocular motor manifestations, nystagmus, neurodegenerative diseases, diplopia
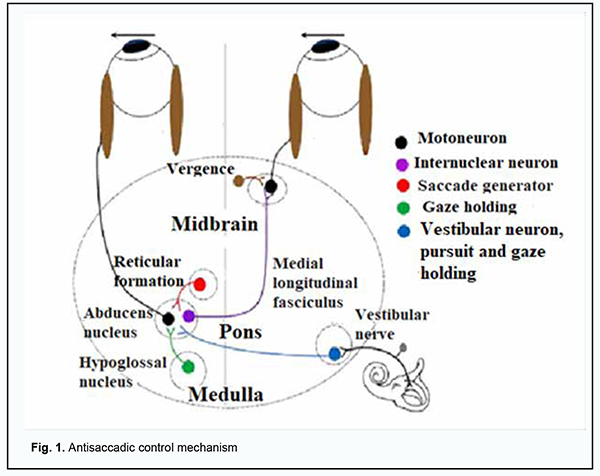
Introduction Neurodegenerative diseases (NDD) are a heterogeneous group of disorders that are characterised by the progressive degeneration of the structure and function of the central nervous system or peripheral nervous system. Vascular dementia, Alzheimer's disease, Parkinson’s disease, and Huntington’s disease are a common cause of morbidity and mortality worldwide, particularly in the elderly [1]. Neurodegenerative diseases can negatively impact the quality of life of affected patients. Because the expected lifespan increases, it is important to elucidate the pathophysiology of NDD in order to improve the potential for diagnostic assessment and therapeutic treatment of these disorders and thus reduce their burden on the healthcare system. Due to its special connection to the brain and its accessibility to measurements, the eye provides a unique window on the brain, thereby offering non-invasive access to a large set of potential biomarkers that might help in the early diagnosis and clinical care of NDD [2]. Oculomotor manifestations (nystagmus and diplopia) do not lead to blindness. However, loss of cognitive abilities in the presence of these manifestations can lead to irreparable social consequences. Laboratory recordings of eye movements can provide valuable information about disease severity, progression or regression in neurodegenerative disease, and hold particular promise for objective evaluation of the efficacy of putative neuroprotective and neurorestorative therapies. For example, saccade performance can be tested to probe both motor and cognitive aspects of oculomotor behavior in Parkinson’s disease, Huntington’s disease vascular dementia, and other neurodegenerative disorders [3]. In Parkinson’s disease, ocular motor abnormalities can be observed in addition to typical motor and non-motor abnormalities [4]. When progressive, the disease impairs the latency and accuracy of visually guided saccades [5]. In addition, compared to healthy controls, patients with Parkinson’s disease have been found to exhibit higher antisaccadic error rates with impaired efficacy of the antisaccade control mechanism (Fig. 1) leading to inhibited reflexive responses and delayed initiation of voluntary saccades [6, 7, 8].
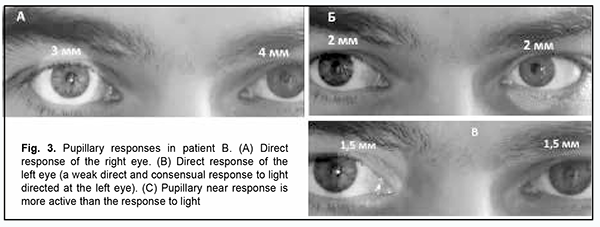
Moreover, internuclear ophthalmoplegia is an ocular motor abnormality which is common in multiple sclerosis (MS) [9]. Impaired saccadic eye movements have been found to be related to clinical performance in MS [10]. Therefore, improved diagnostic assessment of ocular motor abnormalities will enable early detection of neurodegenerative diseases, whereas the correction of these abnormalities will prevent the undesirable consequences. The purpose of this paper was to analyze the nature of oculomotor manifestations of neurodegenerative disorders. Material and Methods Two cases of neurodegenerative disorders are presented, a case of a 30 year-old male with a partial Parinaud's syndrome, and a case of a 52 year-old female with viral encephalomyelitis. Both patients underwent an examination and were consulted at the Department of Ophthalmology, Ivano-Frankivsk National University. They had a routine eye examination which included visual acuity assessment, refractometry, intraocular pressure measurement, visual field assessment, biomicroscopy and ophthalmoscopy. Eye positions and features of ocular motor changes were recorded by photo and video cameras. Brain magnetic resonance imaging (MRI) was performed. Blood and cerebrospinal fluid samples were obtained to perform immunological tests. Informed consent for personal data processing and use for the purposes of research was obtained from both patients. Results Case 1 A 20-year-old male patient presented with complaints of having horizontal incongruous diplopia for more than two years, with the degree and character of double vision experienced depending on (a) the gaze position of the eye and general well being and (b) the fatigue, respectively. He reported that he had polysinusitis and received maxillary and sphenoid sinus surgery a year before presentation. On examination, visual acuity was 1.0 OU, there was bilateral esotropia, and bilateral esophoria of 7 prism diopters. In addition, the patient showed apparent nystagmus at near (Fig. 2). Pupillary examination showed anisocoria. The diameters of the left and right pupils were 3 mm and 4 mm, respectively, in response to light directed at the right eye, indicating a weak direct and consensual response. Both pupils constricted to 2 mm in response to light directed at the left eye. In addition, both pupils constricted to 1.5 mm with gaze shift to near object (Fig. 3). These findings indicated pupillary light-near dissociation.
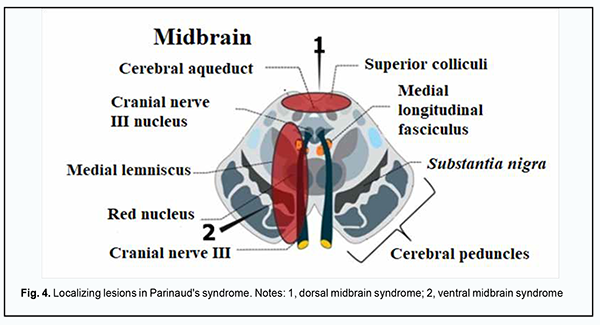
There was mild retraction of the left upper eyelid on downgaze. The patient was diagnosed with a partial Parinaud's syndrome, which was confirmed by MRI evidence of multiple small degenerations at the dorsal-medial pons. Case 2 A 52-year-old female patient presented with complaints of experiencing flashes in her eyes during distant gaze for more than a year. As a consequence of this disorder, the patient had to give up driving, experienced problems with using a computer, lost her reading ability, and began losing her ability to recognize familiar faces. This led to the question wheather she had to give up her career. On examination, the best-corrected visual acuity was 1.0, there was a mild myopia. In addition, there was substantial bilateral vertical rotational nystagmus, more pronounced in abduction. Cerebrospinal fluid samples were obtained to perform immunological and viral tests, and the Epstein-Barr virus was detected. The patient was diagnosed with viral encephalomyelitis. Discussion We presented two cases with ocular motor abnormalities. Case 1 was characterized by a combination of bilateral esotropia, nystagmus at near, pupillary light-near dissociation and retraction of the left upper eyelid on downgaze. The patient was diagnosed with a partial Parinaud's syndrome because the Parinaud syndrome (Fig. 4) is characterized by the following: upgaze paralysis (which was absent in our patient), convergence retraction nystagmus, pupillary light-near dissociation (B), and bilateral upper lid retraction [11, 12].
A positive test for Epstein-Barr virus was consistent with impaired internuclear links in the midbrain. The patient was prescribed prism correction for diplopia. Case 2 was characterized by vertical rotational nystagmus, which is likely to indicate impaired internuclear links in cerebral antisaccadic control. Because these alterations are also characteristic for parkinsonism, the case requires further study. Conclusion The pattern and nature of ocular motor abnormalities are helpful in localizing lesions of such deep brain structures as the brainstem and pons. A more thorough study will be beneficial for early diagnostic assessment and timely treatment of neurodegenerative lesions, and, consequently, for preventing undesirable social consequences.
References 1.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018 Apr 2;10(4):a033118. 2.Guidoboni G, Sacco R, Szopos M, et al. Neurodegenerative Disorders of the Eye and of the Brain: A Perspective of Their Fluid-Dynamical Connections and the Potential of Mechanism-Driven Modeling. Front Neurosci. 2020 Nov 12;14:566428. 3.Anderson T, Mac Askill M. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013 Feb;9(2):74-85. 4.Terao Y, Fukuda H, Ugawa Y, Hikosaka O. New perspectives of the pathophysiology of Parkinson’s disease as assessed by saccade performance: A clinical review. Clin Neurophysiol. 2013 Aug;124(8):1491-506. 5.Terao Y, Fukuda H, Yugeta A, et al. Initiation and inhibitors control of saccades with the progression of Parkinson’s disease: Changes in three major drives converging on the superior colliculus. Neuropsychologia. 2011;49(7):1794–1806. 6.Antoniades CA, Demeyere N, Kennard C, et al. Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: the discovery study. Mov Disord. 2015 May;30(6):843-7. 7.Coe BC, Munoz DP. Mechanisms of saccade suppression revealed in the anti-saccade task. Philos Trans R SocLond B Biol Sci. 2017 Apr 19;372(1718):20160192. 8.Kunimatsu J, Tanaka M. Roles of the primate motor thalamus in the generation of antisaccades. J Neurosci. 2010 Apr;30(14):5108-17. 9.Nij Bijvank JA, LJ Van, LJ Balk, et al. Diagnosis and quantifying a common deficit in multiple sclerosis: internuclear ophthalmoplegia. Neurology. 2019 May 14;92(20):e2299-e2308. 10.Nij Bijvank JA, EM Strijbis, Nauta IM, et al. Impaired saccadic eye movements in multiple sclerosis are related to altered functional connectivity of the oculomotor brain network. Neuroimage Clin. 2021;32:102848.. 11.Goldenberg-Cohen N, Haber J, Ron Y, et al. Long term ophthalmological follow up of children with Parinaud syndrome. Ophthalmic Surg Lasers Imaging. Jul-Aug 2010;41(4):467-71. 12.Feroze KB, Patel BC. Parinaud Syndrome. Treasure Island (FL): StatPearls Publishing; 2019 Jan.
Disclosures Conflict of Interests: The author certifies that there is no conflict of interest that could influence her opinion regarding the subject or materials of this manuscript. Sources of Support: None.
|