J.ophthalmol.(Ukraine).2022;4:49-57.
|
http://doi.org/10.31288/oftalmolzh202244957 Received: 19.05.2022; Accepted: 14.07.2022; Published on-line: 24.08.2022 Recurrent ocular toxoplasmosis infection in a patient with a selective deficiency of NK T-cells and cytotoxic СD8+ T-cells associated with a genetic folate cycle deficiency D. V. Maltsev, O. O. Hurzhii 1 Research Institute of Experimental and Clinical Medicin, Bogomolets National Medical University; Kyiv (Ukraine) 2 Visium Clinic; Kyiv (Ukraine) TO CITE THIS ARTICLE: Maltsev DV, Hurzhii OO. Recurrent ocular toxoplasmosis infection in a patient with a selective deficiency of NK T-cells and cytotoxic СD8+ T-cells associated with a genetic folate cycle deficiency. J.ophthalmol.(Ukraine).2022;4:49-57. http://doi.org/10.31288/oftalmolzh202244957
This paper reports a case of recurrent toxoplasmic chorioretinitis in a patient with cellular immunodeficiency. A 37-year-old male presented to an ophthalmologist with complaints of reduced visual acuity and discomfort in his left eye. He had a history of at least two episodes of acute posterior uveitis without identifying the cause of inflammation. An ophthalmoscopic evaluation revealed a scar in the right retina and signs of acute vitritis and chorioretintis surrounding a scar in the left retina. Paired serology confirmed a diagnosis of toxoplasmosis. A deficiency of NK T-cells and cytotoxic СD8+ T-cells was noted, but there was no evidence of secondary immunosuppression. The Primary Immunodeficiency (PID) panel providing sequencing of 208 genes did not find a disease. A test for genetic folate cycle deficiency was conducted due to persistent hyperhomocysteinemia. The genetic testing identified two pathogenic polymorphisms in the genes coding for folic acid cycle enzymes (heterozygous MTHFR A1298C and homozygous MTRR A66G), which was believed to be associated with a cellular immunity, taking into account the data on immunosuppression and opportunistic infections in the presence of a genetic folate cycle deficiency. The following treatment was administered: spiramycin, 3.0 mln units orally daily for 14 days, to inhibit toxoplasma; recombinant human alpha2 interferon (3.0 mln units intramuscularly every other day for a month) and oxodihydroacridinylacetate sodium 2.0 mln units intramuscularly every other day for a month, with switching between this agent and interferon, to compensate for a deficiency of NK T-cells and СD8+ T-cells; and daily peribulbar injection of betamethasone 4 mg/mL for 3 days. The first signs of improved visual acuity were seen at day 8, and a complete restoration of vision in the left eye was achieved by the end of one month of combination therapy. In addition, the patient received three one-month courses of alpha2 interferon for compensation of cellular immunodeficiency over two years which prevented a recurrence of toxoplasmosis. Keywords: toxoplasmic chorioretinitis, vitritis, immunodeficiency, immunotherapy
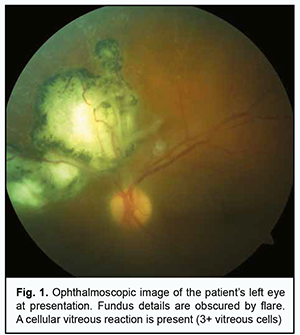
Introduction Clinical management of eye patients with severe recurrent ocular lesions from opportunistic infections is a challenge because it requires close cooperation of a wide range of medical specialties for identification of the infectious agent, rational assessment of the immune status, search for the cause of immunosuppression, and delivery of comprehensive therapy that would include not only measures to suppress microbial infection but also those to compensate for an immune dysfunction that has caused reactivation of latent or persistent infection in the human body. Toxoplasma gondii is a typical opportunistic agent which is re-activated in the body of an immunocompromised individual with a primary or secondary immunodeficiency, and can cause severe ocular lesions in humans. In their recent overview on clinical aspects of ocular toxoplasmosis, Fabiani and colleagues [1] noted that at least 30% of current population are seropositive for Toxoplasma gondii, and it is in this cohort of individuals that severe ocular lesions can develop if the parasite reactivates under conditions of immunosupression. Kalogeropoulos and co-authors [2] have reviewed recently the current diagnostic and therapeutic approaches to Toxoplasma gondii, and reported that, although the prevalence has somewhat decreased over a recent decade, the agent is still a major cause of posterior uveitis in individuals with low immunity, which manifests itself as vitritis and chorioretinitis. Because cases of severe ocular toxoplasmosis infection have been reported mostly in patients with HIV infection [3, 4], malignancies [5] and primary immunodeficiency [6], immune status assessment is essential for a rational differential diagnostic approach in clinically manifest toxoplasmosis in humans. Our literature review found that reports on the reactivation of toxoplasmosis in immunocompetent individuals are extremely rare [7]. In this paper, we describe a case of recurrent toxoplasmic chorioretinitis in cellular immunodeficiency and demonstrate the importance of assessing for the immune status and selecting the targeted immunotherapy for removing the signs of immunosuppression in toxoplasmosis. The patient signed informed consent regarding participation in the study and the processing of personal data. The study adhered to the Declaration of Helsinki. Case report A 37-year-old male presented to an ophthalmologist with complaints of reduced visual acuity and discomfort in his left eye. He had a history of at least two episodes of acute posterior uveitis without identifying the cause of inflammation and, consequently, without administering a causal medical therapy and addressing the underlying etiology. The patient reported that those episodes had been treated with non-specific corticosteroid anti-inflammatory therapy in the form of periocular injections and topical eye drops. On eye examination at presentation, Snellen visual acuity in the right eye was 20/20. There was no biomicroscopic or ophthalmoscopic evidence of active inflammation in the right eye. Ophthalmoscopically, the right optic disc appeared pale pink with clear margins. Macular reflex was clear, and the macular region showed no pathological changes. There was a white lesion measuring 0.1 disc diameter with clear pigmented margins in the temporonasal paramacular region. The vitreous above the lesion showed no pathological changes. At presentation, Snellen visual acuity in the left eye was 20/100. Biomicroscopy of the left eye showed clear aqueous and no cells or fibrinous effusion in the aqueous. Papillary reaction to light was normal. Ophthalmoscopy of the left eye showed an inflammatory cell response and floating clumps of inflammatory cells in the vitreous body (Fig. 1).
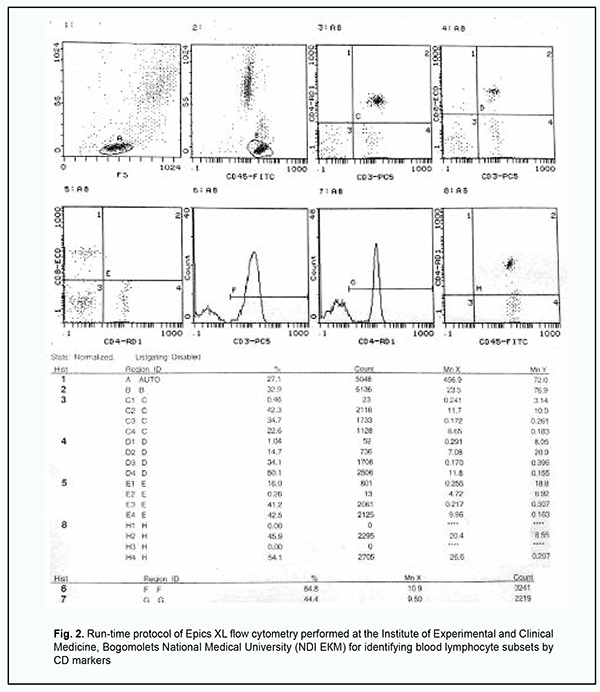
In the peripapillary region there were white retinal lesions (measuring as much as 7-8 disc diameters totally) with sharp pigmented margins (Fig. 1). In addition, below these lesions, there was a peripapillary white lesion with blurred margins and increased cell infiltration above the lesion area. White perivascular sleeves surrounding the lesion were observed in the retina. These ophthalmoscopic findings were interpreted according to Stokkermans and Havens in their recent study on human Toxoplasma retinochoroiditis [8]. Blood white cell PCR and eye swab PCR with specific primers for herpes simplex virus (HSV) 1/2, Varicella-zoster virus (VZV) , Epstein Barr virus (EBV), Cytomegalovirus (CMV), human herpesvirus (HHV)-6, HHV-7, HHV-8, adenoviruses, enteroviruses, torque teno virus (TTV), B19 parvovirus, hepatitis В, С, D and G, T. gondii, Borrelia burg., Chlamydia pneum., and Mуcoplasma pneum were performed at the Neurobiochemistry Laboratory of the Romodanov Neurosurgery Institute to find the cause of acute chorioretinitis that was believed to be recurrent given the old chorioretinal scars in both eyes. All PCR tests were negative. Serum levels of specific IgM and IgG to the above microbial agents were determined at the Neurobiochemistry Laboratory. Serological studies identified increased serum levels of specific IgG to T. gondii (324 IU/ml against a normal value of N˂10 IU/ml) and CMV (84 IU/ml against a normal value of N˂10 IU/ml), but not to other causative agents. At this time point, serum levels of specific IgG to T. Gondii were found to be increased more than fourfold (324 IU/ml against 63 IU/ml), whereas those to CMV, were found not to change substantially (84 IU/ml against 76 IU/ml), compared to 3 months before (the time point of previous exacerbation of ocular pain). Therefore, paired serology confirmed a Toxoplasmic etiology, but not a CMV etiology of acute chorioretinitis in the left eye. Zhang and colleagues [9] overviewed the serological diagnosis of toxoplasmosis, including diagnostic strategy, current problems in detection with specific antibodies, and the standardization of T. Gondii serological detection. They reported that, in the clinic, the serological detection of IgМ and IgG antibody levels are the basis for identifying infection and the most commonly used methods. Particularly, the identification of specific IgM antibodies or evidence of an increase in the titer of specific IgG antibodies over a short period after initial testing indicates reactivation of the pathogen. Chan and colleagues [10] reported on the results of a National Serosurvey in Haiti, and concluded that multiplex serological assays can provide a wealth of information about population exposure to different infectious diseases including toxoplasmosis. Borges and co-authors [11] compared the detection of immunoglobulin isotypes and subclasses against Toxoplasma gondii soluble antigen in serum and colostrum samples from puerperal women, and reported that serum and colostrum immunoglobulins of different classes and subclasses provide a wealth of information about exposure to toxoplasmosis. A recent case-control study by Babekir and colleagues [12] evaluated the association of T. gondii infection and liver disease, and found serum T. gondii IgG+ antibody to be significantly associated with liver injury biomarkers and an increased risk of chronic liver disease and nonalcoholic fatty liver disease, demonstrating the value of serological tests in toxoplasmosis. Because Toxoplasma infection is caused by an opportunistic agent which is reactivated from latency or persistent infection mostly under conditions of immunosuppression, we believed it was reasonable to assess the immune status of the patient in order to elucidate a causative immunodeficiency. Another reason for this belief was that, in the case reported here, the presence of old chorioretinal scars in both eyes was consistent with the recurrence of toxoplasmic chorioretinitis. Immunological evaluation included complete blood cell count and lymphocyte subpopulation analysis by flow cytometry on a Beckman Coulter Epics XL flow cytometer (Beckman Coulter, Miami, Fla.) and indirect immunofluorescence with monoclonal antibodies to CD markers with two or three immunofluorescent labels (CD3+ (N = 54–83%), CD3+CD4+ (N = 26–58%), CD3+CD8+ (N = 21–35%), CD3–CD19+ (N = 5–14%), CD3–CD16+CD56+ (N = 5–15%), CD3+CD16+CD56+(N = 3–8%)) and calculation of the immunoregulatory index (N = 1.2 – 2.3) (reagents from Beckman Coulter). The run-time protocol of Epics XL (Epics XL- Flow Cytometer; Beckman Coulter Inc., Brea, CA) flow cytometry for identifying blood lymphocyte subsets by CD markers is presented in Fig. 2).
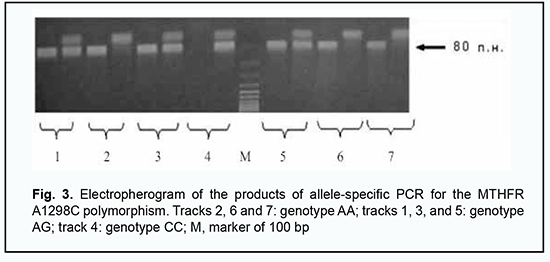
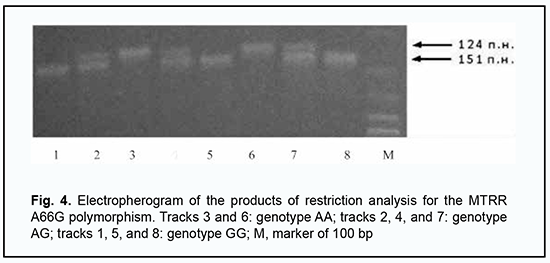
Functional T cell activity was assessed by concanavalin A-induced T cell blast transformation (N = 1.2 – 1.68 optical units). Phagocytosis was assessed by latex bead ingestion with the determination of the phagocytic index (N = 1.5–3.0 units) and by the myeloperoxidase activity (ELISA, N = 18–23 units) and NADPH oxidase activity of neutrophils (nitroblue tetrazolium test; spontaneous activity, N = 80–125 units; induced activity, N = 150–380 units). Serum levels of major immunoglobulin classes were determined by enzyme-linked immunosorbent assay (ELISA) (VektorBEST, Russia; N IgM = 0.8–1.6 g/L, N IgA = 0.6–2.5 g/L, N IgG = 6.0–15.0 g/L). Serum levels of minor IgE (N = 30–100 IU/mL), IgD (N ˃ 13 IU/mL) and IgG (N IgG1= 280–1120 mg per scale division, N IgG2 =30–630 mg per scale division, N IgG3 = 40–250 mg per scale division, N IgG4 = 11–620 mg per scale division) immunoglobulin classes were determined by ELISA (VektorBEST, Russia; MDI Limbach Berlin GmbH, Germany). The results of a complete blood cell count indicated absolute lymphocytosis and monocytosis and relative neutropenia in the presence of a normal blood sedimentation rate. We found that, of the immune status biomarkers assessed, only the counts of CD3+CD16+CD56+ T-cell (natural killer T-cell) and cytotoxic СD8+ T-cells were not within the reference ranges; particularly, they were almost less than half of the low limit of the normal range (1.4%; 0.02 х 109/L and 12%; 0.08 х 109/L, respectively). To further validate our findings, we randomly repeated immunological studies and obtained similar results (1.3%; 0.04 х 109/L and 13%; 0.09 х 109/L, respectively) to initial studies. Therefore, we identified a selective deficiency of NK T-cells and cytotoxic СD8+ T-cells. These findings are in agreement with the current ideas on the mechanisms of immune surveillance of T. gondii, with a subset of cytotoxic NK T-cells having been attributed a key role in an effector immune response to the opportunistic pathogen [13]. The results of at least three recent reviews of clinical studies on immune protection in human toxoplasmosis have demonstrated key importance of cytotoxic СD8+ and NK T-cells in the control over the latent Toxoplasma in the human body [14, 15, 16]. It was obvious that deficiency of NKT cells and cytotoxic СD8+ T cells and was the most likely cause of a critical impairment in immune surveillance of endogenous T. gondii and the development of T. gondii-induced acute chorioretinitis. However, the question of the origin of the above cellular immunodeficiency in our patient was still open. The patient’s history and additional studies gave no evidence of such common causes of secondary immunosuppression as HIV infection, endocrine disorder, malignancies and taking immunosuppressive medications. We had to exclude primary immunodeficiency whose laboratory phenotype could include deficiency of NKT cells and cytotoxic СD8+ T cells. For this purpose, Primary Immunodeficiency (PID) panel (Centogene, Rostock, Germany) was employed, including studies of nucleotide variants of more than 208 genes associated with well-known human primary immunodeficiencies. Since these genetic studies were negative, we retrospectively analyzed the results of all the available biochemical studies that the patient had undergone throughout his life. In this way we found a characteristic pathologic phenomenon, hyperhomocysteinemia (i.e., pathologically increased serum homocysteine levels); this was seen in the results of numerous studies which the patient had undergone. Thus, the patient’s serum homocysteine levels varied from 11.6 µMol/L to 19.1 µMol/L against a normal value of < 8 µMol/L. This finding supposed a genetic folate cycle deficiency, because hyperhomocysteinemia is a characteristic laboratory finding of this genetic disorder [17, 18]. Consequently, the patient had a special genetic testing at the Neurobiochemistry Laboratory of the Romodanov Neurosurgery Institute to identify pathologic polymorphisms in the genes coding for folic acid cycle enzymes, MTHFR C677T (rs1801133), MTHFR A1298C (rs1801131), MTRR A66G (rs1801394) and MTR A2756G (rs1805087). The genetic testing identified two pathogenic polymorphisms in the genes coding for folic acid cycle enzymes (heterozygous MTHFR A1298C and homozygous MTRR A66G), which was consistent with the condition of persistent hyperhomocysteinemia, and confirmed the presence of a genetic folate cycle deficiency in the patient. The results of restricted PCR for pathogenic polymorphisms in MTHFR and MTRR genes are presented in Figs. 3 and 4.
Experimental and clinical studies have reported on alterations of the immune status in patients with a verified genetic folate cycle deficiency as well in those with a folic acid deficiency. Van der Weyden and colleagues [19] found a suppressed metabolism of lymphoblasts in folate deficiency which involves altered deoxynucleotide metabolism and thymidylate cycle activities. Partearroyo and co-authors [20] demonstrated that vitamin B12 and folic acid imbalance typical for the folate cycle deficiency phenotype suppressed the NK function and B lymphocyte activity and induced lymphoproliferation. Courtemanche and colleagues [21] found that folate deficiency inhibited the proliferation of primary human cytotoxic CD8+ T lymphocytes. Abe and co-authors [22] demonstrated that folic acid deficiency resulted in a reduction in the number of NK cells, T lymphocytes and B cells, but not in the number of basophils and granulocytes. Unmetabolized folic acid in serum has been noted in genetic folate cycle deficiency and was found by Troen and colleagues [23] to be associated with reduced NK cell cytotoxicity among postmenopausal women. Bhatnagar and colleagues [24] described pancytopenia due to severe folate deficiency. In addition, it has been reported previously on the development of severe opportunistic infections due to NK deficiency in patients with genetic folate cycle deficiency. Particularly, HSV-2-associated transverse sacral myelitis has been reported in a patient with selective NK cell deficiency associated with genetic folate cycle deficiency [25]. We have previously reported [26] on a specific combined immune deficiency of cytotoxic NK cells, NKT cells, CD8+ T cells, and phagocytic myeloperoxidase and dysimmunoglobulinemia, and associated with pathological polymorphisms in the genes coding for folic acid cycle enzymes in a case-control study of children with autism spectrum disorders. Another case-control study demonstrated that the above deficiency in children resulted in the formation of a special microbial spectrum including a number of opportunistic and potentially pathogenic infectious agents. Particularly, TTV was found in 87%, HHV-7 in 79%, HHV-6 in 68%, EBV in 59%, Streptococcus pyogenes in 46%, Candida albicans in 41%, Borrelia in 34%, Mycoplasma pneumoniae in 27%, Chlamydia pneumoniae in 26%, Yersinia enterocolitica in 23%, and T. gondii in 19% of cases [27]. Altogether, these findings made us believe that a deficiency of NK T-cells and СD8+ T-cells in the case reported here was caused by genetic folate cycle deficiency. Therefore, the patient was clinically diagnosed with genetic folate cycle deficiency (MTHFR A1298C hetero, MTRR A66G homo): hyperhomocysteinemia: selective deficiency of NK T-cells and СD8+ T-cells: and acute recurrent toxoplasma chorioretinitis. The clinical diagnosis statement represented the most likely scenario of pathological events with obvious cause-and-effect relations between the detected laboratory and clinical phenomena in the patient and enabled administer the following treatment components: spiramycin, 3.0 mln units orally daily for 14 days, to inhibit toxoplasma; recombinant human alpha2 interferon (3.0 mln units intramuscularly every other day for a month) to compensate for a deficiency of NK T-cells and СD8+ T-cells, as per the findings of clinical studies by Yamagiwa and colleagues [28] and Okumura and colleagues [29]; oxodihydroacridinylacetate sodium (an inducer of endogenous interferon synthesis) 2.0 mln units intramuscularly every other day for a month, with switching between this agent and interferon, to compensate for a deficiency of NK T-cells and СD8+ T-cells, as per the findings of a relevant clinical study [30]; and daily peribulbar injection of betamethasone 4 mg/mL for 3 days. Therefore, the patient was receiving a combination treatment aimed not only at inhibiting toxoplasma (by spiramycin), but also at compensating for a cellular deficiency (by alpha2 interferon and an inducer of interferon synthesis), the deficiency with which, most likely, the reactivation of the parasite was associated, and recurrent toxoplasma invasion had been associated. The selection of recombinant human alpha2 interferon was caused by case-control study findings [28, 29] pointing to the ability of this immunotherapeutic agent to normalize low numbers of NK T-cells and СD8+ T-cells in immunocompromised patients. Moreover, this selection was consistent with the data of a recent review [31] of clinical studies on toxoplasmosis which demonstrated that it is interferon-dependent mechanisms of immune modulation that are key players in the control of cellular immunity, which provides immune surveillance of latent T. gondii. The first signs of improved visual acuity were seen at day 8, and a complete restoration of vision was achieved by the end of one month of combination therapy. One month after completion of the above course of treatment, there was ophthalmoscopic evidence of both the absence of vitreous inflammatory cell reaction and the presence of dense fibrous vitreous strands at the area of chorioretinal lesions. There were areas of increased pigmentation at the margins of chorioretinal lesions, whereas the lesion with previously blurred margins became clearly outlined, showing some pigment deposits. A repeat immunological examination revealed normal numbers of NK T-cells and СD8+ T-cells in the peripheral blood, indicating that a causative immune deficiency has been compensated for and giving hope for restoration of immune surveillance of endogenous T. gondii. Subsequently, the patient had regular medical check-ups at ophthalmologist and clinical immunologist over two years. During this period, he received three additional one-month courses of immunotherapy with recombinant human alpha2 interferon (3.0 mln units intramuscularly every other day for a month) to compensate for a renewed NK T-cell and СD8+ T-cell deficiency noted at immunological check-up examinations. We believe that it is due to this strategy of preventive targeted immunotherapy for cellular deficiency that a subsequent recurrence of severe ocular toxoplasmosis infection has been avoided. Serum levels of specific IgG to T. gondii decreased almost 7-fold (to 47 IU/ml against a normal value of ˂10 IU/ml) and reached levels > 4-fold the upper reference range over this period, which is consistent with the ideas of immune memory to a latent pathogen that has not been reactivated for a long time. Discussion This case demonstrates the benefit from close cooperation between ophthalmologists and clinical immunologists involved in managing immunocompromised patients with recurrent ocular lesions from opportunistic infections. Special immunological and genetic studies not only allow detecting an immunosuppressive condition that contributes to reactivation of an endogenous opportunistic infectious agent from latency or persistent infection but also allow administering the targeted immunotherapy that compensates for a causative immunodeficiency and, in this way, improves the outcome of antimicrobial treatment and prevents a further severe recurrence of reactivated opportunistic infection. We have previously reported on other cases of successful cooperation between ophthalmologists and clinical immunologists involved in managing patients with an immunocompromised system and associated ocular lesions. Particularly, we have reported on recurrent Toxoplasma chorioretinitis in a young female patient with primary myeloperoxidase deficiency and a benefit from the use of recombinant human gamma interferon as a basic immunotherapeutic treatment for her phagocytic abnormality [32]. In addition, we have reported on a case of ANA-associated uveitis in the presence of reactivated HHV-7 infection in a patient with primary mannose binding lectin deficiency and the efficacy of replacement therapy with cryopreserved human plasma for compensation of the causative immune dysfunction [33]. In another patient [34], the primary mannose binding lectin deficiency was associated with persistent recurrent and resistant to recommended antiviral drugs herpes zoster ophthalmicus infection, and only administration of the basic immunotherapy with cryopreserved human plasma for a complement system deficiency allowed achieving persistent remission of the recurrent opportunistic viral infection. We reviewed PubMed for other reports on reactivation of severe toxoplasmosis infection in the presence of primary immunodeficiency in humans. It has been reported on reactivation of toxoplasmosis infection in common variable immunodeficiency [35], Louis-Bar syndrome [6], X-linked hyper IgM syndrome [36], primary T-cell immunodeficiency associated with defective transmembrane calcium influx [37], hypomorphic mutation in the CD40 ligand gene [38], Good's syndrome [39], PI3-Kinase delta Syndrome Type 2 [40] and warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) [41]. There are, however, primary immmunodeficiencies which paradoxically decrease the risk of reactivation of toxoplasmosis infection or make the disease run a slow course. Particularly, Meyer and colleagues [42] have found that progression to toxoplasmosis as a first AIDS-defining illness was significantly delayed in individuals with CCR5-delta32 deletion, but protective effects of primary immmunodeficiencies in Toxoplasma invasion are the exception rather than a typical clinical phenomenon. With this report, we are expanding a spectrum of publications on severe opportunistic ocular infections in patients with primary minor immunodeficiency, and one more time demonstrating the obvious benefit of well-planned immunological studies and targeted immunotherapy in these cases. Conclusion Patients with recurrent ocular lesions caused by opportunistic infections (e.g., toxoplasmosis) should (a) undergo an immunological examination to find a cause of reactivation of a latent opportunistic agent and (b) receive not only antimicrobial therapy but also immunotherapeutic interventions to correct for the immune status. This will allow not only arresting an acute infection event, but also preventing a further recurrence.
References 1.Fabiani S, Caroselli C, Menchini M, et al. Ocular toxoplasmosis, an overview focusing on clinical aspects. Acta Trop. 2022 Jan;225:106180. 2.Karanovic D, Michelow IC, Hayward AR, et al. Disseminated and Congenital Toxoplasmosis in a Mother and Child With Activated PI3-Kinase delta Syndrome Type 2 (APDS2): Case Report and a Literature Review of Toxoplasma Infections in Primary Immunodeficiencies. Front Immunol. 2019 Feb 14;10:77. 3.de-la-Torre A, Gómez-Marín J. Disease of the Year 2019: Ocular Toxoplasmosis in HIV-infected Patients. Ocul Immunol Inflamm. 2020 Oct 2;28(7):1031-1039. 4.Srivastava S, Kundu A, Sivakumar H, et al. A case of Toxoplasma gondii and Strongyloides stercoralis coinfection in an immunocompromised patient. Infect. Disord Drug Targets. 2022 Feb 18. Online ahead of print. 5.Parasram M., Arevalo-Perez J. Cerebral toxoplasmosis in a patient with multiple myeloma. Surg Neurol Int. 2022 May 6;13:191. 6.Gioia LV, Bonsall D, Moffett K, et al. Bilateral maculopathy in a patient with ataxia telangiectasia. J AAPOS. 2016 Feb;20(1):85-8. 7.Nelwan EJ, Shakinah S, Clarissa G, et al. Rare cardiac complication of toxoplasmosis in immunocompetent host. IDCases. 2022 Jun 15; 29: e01533. 8.Stokkermans TJ, Havens SJ. Toxoplasma Retinochoroiditis. [Updated 2022 Mar 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 9.Zhang K, Lin G, Han Y, Li J. Serological diagnosis of toxoplasmosis and standardization. Clin Chim Acta. 2016 Oct 1;461:83-9. 10.Chan Y, Martin D, Mace KE, et al. Multiplex Serology for Measurement of IgG Antibodies Against Eleven Infectious Diseases in a National Serosurvey: Haiti 2014-2022. Front Public Health. Jun 9; 10: 897013. 11.Borges HDS, Oliveira-Scussel ACM, Oliveira ÂMM, et al. Comparative Detection of Immunoglobulin Isotypes and Subclasses against Toxoplasma gondii Soluble Antigen in Serum and Colostrum Samples from Puerperal Women. Int J Environ Res Public Health. 2022 Jun 29; 19(13): 7953. 12.Babekir A., Mostafa S., Minor R.C. et al. The Association of Toxoplasma gondii IgG and Liver Injury in US Adults. Int J Environ Res Public Health. 2022 Jun 19;19(12):7515. 13.Combe CL, Curiel TJ, Moretto MM, Khan IA. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect Immun. 2005 Aug;73(8):4913-21. 14.Khan I.A., Moretto M. Immune responses to Toxoplasma. Curr Opin Immunol. 2022 Jul 1 ;77: 102226. 15.Sana M, Rashid M, Rashid I, et al. Immune response against toxoplasmosis-some recent updates RH: Toxoplasma gondii immune response. Int J Immunopathol Pharmacol. Jan-Dec 2022; 36: 3946320221078436. 16.Sasai M, Yamamoto M. Anti-Toxoplasma host defense systems and the parasitic counterdefense mechanisms. Parasitol Int. 2022 Aug;89:102593. 17.Li WX, Cheng F, Zhang AJ, et al. Folate Deficiency and Gene Polymorphisms of MTHFR, MTR and MTRR Elevate the Hyperhomocysteinemia Risk. Clin Lab. 2017 Mar 1;63(3):523-533.Crossref 18.Zhang L, Yuan XF, Li Q, et al. Correlation between Serum Homocysteine Level, MTHFR Gene Polymorphism and Patients with Hematological Diseases Complicated with Coronary Heart Disease. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022 Feb;30(1):305-309 19.Van der Weyden MB, Hayman RJ, et al. Folate-deficient human lymphoblasts: changes in deoxynucleotide metabolism and thymidylate cycle activities. Eur J Haematol. 1991 Aug;47(2):109-14. 20.Partearroyo T, Úbeda N, Montero A. Vitamin B(12) and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoprolipheration in aged rats. Nutrients. 2013 Nov 26;5(12):4836-48. 21.Courtemanche C, Elson-Schwab I, Mashiyama ST. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004 Sep 1;173(5):3186-92. 22.Abe I, Shirato K, Hashizume Y. Folate-deficiency induced cell-specific changes in the distribution of lymphocytes and granulocytes in rats. Environ Health Prev Med. 2013 Jan;18(1):78-84. 23.Troen AM, Mitchell B, Sorensen B. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006 Jan;136(1):189-94. 24.Bhatnagar N, Wechalekar A, McNamara C. Pancytopenia due to severe folate deficiency. Intern Med J. 2012 Sep;42(9):1063-4. 25.Maltsev DV, Gorbenko VYu. [HSV-2-associated transverse sacral myelitis in a patient with selective natural killer cell deficiency: a case report]. Ukr Neurol Zh. 2018; №2: 74–80. Ukrainian. 26.Maltsev DV. Features of folate cycle disorders in children with ASD. Bangladesh J Med Sci. 2020; Vol. 19(4): 737–742. 27.Maltsev DV. [The results of the study of the microbial spectrum in children with autism spectrum disorders associated with genetic deficiency of the folate cycle]. Choloviche zdorov’ia, genderna ta psyhosomatychna medycyna. 2021; № 1-2: 26–39. Ukrainian. 28.Yamagiwa S, Matsuda Y, Ichida T, et al. Sustained response to interferon-alpha plus ribavirin therapy for chronic hepatitis C is closely associated with increased dynamism of intrahepatic natural killer and natural killer T cells. Hepatol Res. 2008 Jul;38(7):664-72. 29.Okumura A, Ishikawa T, Maeno T, et al. Changes in natural killer T cells subsets during therapy in type C hepatitis and hepatocellular carcinoma. Hepatol Res. 2005 Aug;32(4):213-7. 30.Maltsev DV. [New discoveries in the mechanisms of interferon-dependent control of latent alpha-herpes virus in sensory ganglia and the experience of using an inducer of interferon overin for the restoration of such control]. Choloviche zdorov’ia, genderna ta psyhosomatychna medycyna. 2018; № 2: 19–32. Ukrainian. 31.Skariah S, Sultan AA., Mordue DG. IFN-induced cell-autonomous immune mechanisms in the control of intracellular protozoa. Parasitol Res. 2022 Jun;121(6):1559-1571. 32.Maltsev DV, Hurzhii OO. Toxoplasma chorioretinitis in primary myeloperoxidase MPO deficiency: A case report. J Ophthalmol (Ukraine). 2019; Vol. 4: 75-81. 33.Maltsev DV, Hurzhii OO. ANA-associated uveitis in the presence of reactivated HHV-7 infection in a patient with MBL deficiency. J Ophthalmol. (Ukraine). 2020; Vol. 6 (497): 64–9. 34.Maltsev DV. A case of persistent recurrent herpes zoster ophthalmicus in a patient with primary mannose binding lectin deficiency. J Ophthalmol (Ukraine). 2021; Vol. 6 (503): 64–68. 35.Shachor J, Shneyour A., Radnay J. et al. Toxoplasmosis in a patient with common variable immunodeficiency. Am J Med Sci. May-Jun 1984;287(3):36-8. 36.Liu X, Zhou K, Yu D, et al. A delayed diagnosis of X-linked hyper IgM syndrome complicated with toxoplasmic encephalitis in a child: A case report and literature review. Medicine (Baltimore). 2017 Dec;96(49):e8989. 37.Le Deist F, Hivroz C, Partiseti M, et al. A primary T-cell immunodeficiency associated with defective transmembrane calcium influx. Blood. 1995 Feb 15;85(4):1053-62. 38.Yong PF, Post FA, Gilmour KC, et al. Cerebral toxoplasmosis in a middle-aged man as first presentation of primary immunodeficiency due to a hypomorphic mutation in the CD40 ligand gene. J Clin Pathol. 2008 Nov;61(11):1220-2. 39.Sasson SC, Davies S, Chan R, et al. Cerebral toxoplasmosis in a patient with myasthenia gravis and thymoma with immunodeficiency/Good's syndrome: a case report. BMC Infect Dis. 2016 Aug 30;16(1):457. 40.Kalogeropoulos D, Sakkas H, Mohammed B, et al. Ocular toxoplasmosis: a review of the current diagnostic and therapeutic approaches. Int Ophthalmol. 2022 Jan;42(1):295-321. 41.McDermott DH, Heusinkveld LE, Zein WM, et al. Case Report: Ocular toxoplasmosis in a WHIM syndrome immunodeficiency patient. F1000Res. 2019 Jan 2;8:2. 42.Meyer L, Magierowska M, Hubert JB, et al. CCR5 delta32 deletion and reduced risk of toxoplasmosis in persons infected with human immunodeficiency virus type 1. The SEROCO-HEMOCO-SEROGEST Study Groups. J Infect Dis. 1999 Sep;180(3):920-4.
Disclosures Received 19.05.2022 Accepted 14.07.2022 Corresponding Author: Maltsev D.V., email: dmaltsev@ukr.net Conflict of Interests: The author certifies that there is no conflict of interest that could influence his opinion regarding the subject matter or materials described and discussed in this manuscript.
|