J.ophthalmol.(Ukraine).2022;4:40-48.
|
http://doi.org/10.31288/oftalmolzh202244048 Received: 01.07.2022; Published on-line: 24.08.2022 Rat tissue responses to dacarbazine-containing implants made of cross-linked polyurethane of different densities N. A. Galatenko 1, R. A. Rozhnova 1, D. V. Kuliesh 1, V. D. Denisenko 1, A. P. Maletskyy 2, N. M. Bigun 3
1 The Institute of Macromolecular Chemistry of the National Academy of Sciences of Ukraine; Kyiv (Ukraine) 2 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) 3 Lviv Regional Clinical Hospital; Lviv (Ukraine) TO CITE THIS ARTICLE: Galatenko NA, Rozhnova RA, Kuliesh DV, Denisenko VD, Maletskyy AP, Bigun NM. Rat tissue responses to dacarbazine-containing implants made of cross-linked polyurethane of different densities. J.ophthalmol.(Ukraine).2022;4:40-8. Background: Restorative orbital and periorbital surgeries require implantable materials containing antimicrobial and anticancer medications. Purpose: To assess rat tissue responses to dacarbazine-containing implants made of cross-linked polyurethane of different densities. Material and Methods: Dacarbazine (DC)-containing polyurethane-urea foam (PUUF) implants were a subject of research. We studied the dynamics of DC release from these implant samples. Soft tissue cellular responses to the developed composites were assessed through the implantation of the latter in Wistar rats. Results: We found that the number of open pores impacts implant survival. There was no substantial difference in DC release between the PUUF samples with a DC weight percent of 1% and those with a DC weight percent of 0.5% at incubation days 1 to 3. At days 5, 7 and 14, an increase in DC release was higher in the former samples than in the latter samples (64% versus 55%). In animals with a subcutaneously implanted PUUF sample, there was histological evidence of (a) formation of a capsule and tight network of blood vessels surrounding an implanted sample at day 3, and (b) separation of the implanted material from the surrounding tissues by a wide cuff of lymphocytes and a thick connective tissue capsule (CTC) at day 7. Conclusion: The number of open pores impacts DC release and the speed of connective tissue ingrowth into the porous matrix. It was found that a CTC formed around all implants at early time points and at day 30, which in combination with the presence of DC in composite materials resulted in long-term cellular inflammation at the implant placement site. Keywords: cross-linked polyurethane composite implant, dacarbazine deposition and diffusion, cellular response
Introduction Craniofacial injuries represent 29% of all trauma cases [1-3], and are primarily caused by anthropogenic and criminal-related ocular and orbital trauma. In this connection, and after surgery for eye cancer, more and more patients need to have their affected orbit, orbital adnexa, and periorbital area reconstructed or restored [4, 5]. It is noteworthy that it is important to use implant materials containing antimicrobial and anticancer medications to prevent inflammation around the implant or malignancy recurrence after excision. Surgeons are not always satisfied with using biological tissue as a ductile material, and legal requirements for harvesting of donor material have been made increasingly stronger. The development of synthetic polymer materials for restoration of body structures that have suffered anatomical and/or functional damage is therefore of primary importance. Advances in the synthesis of biocompatible polymer materials for medical applications have been reported recently [6, 7]. The implementation of the possibility for endowing a polymeric implant with curative properties as a result of its chemical and physical bonding to biologically active substances has been demonstrated [8-10]. Polymeric chains may be linear, branched to variable degrees, or a polymeric material may have a mesh-like structure. Chemical structure of polymeric materials determines their mechanical and physical-and chemical properties [11-13]. Endowing a polymeric implant with curative properties represents a promising approach in polymer chemistry. The medication not bonded to a polymer molecule gradually diffuses in the body during endoprosthesis implantation, whereas the medication bonded to a polymer molecule is characterized by a longer release into surrounding tissues [14-17]. A short-action implant, while staying within the body, has to undergo permanent destruction-and-replacement by tissue regenerate, taking in account a local drug effect on a particular disease. Of note that the biodegradation of polymeric implant material may depend on material porosity, with different biological effects on surrounding tissues. Since the proposed polyurethane polymers can be used for developing implants capable of depositing medications (e.g., anti-tumor medications), we believed it was feasible to assess (a) diffusion properties of polyurethane polymers of different densities, including polymers with immobilized dacarbazine, and (b) the response of the rat tissue to the above implants. Porosity has effects on biocompatibility and biointegration of implant materials [18]. Open porosity of implants facilitates viable tissue ingrowth into the material, which improves implant survival with a reliable implant placement, and, in some cases, the elastic structure of these systems may contribute to reduced pressure on, and damage to, these tissues. The internal media of the body contributes to biological destruction of polyurethane implants and their quick integration with tissues, whereas a prolonged release of immobilized bioactive substances into surrounding tissues contributes to a mild cellular response and prompt patient rehabilitation [14, 19]. We performed the selection of the optimal component ratio for the synthesis of a series of polyurethane-urea foams with immobilized dacarbazine (as a drug depot) in order to solve the task [19]. The purpose of the study was to assess rat tissue responses to dacarbazine- containing implants made of cross-linked polyurethane of different densities. Material and Methods Polyurethane-urea foams (PUUF) fabricated from a mixture of olygourethan diisocyanates of different molecular masses and containing dacarbazine, a medical substance, at a weight percent of 0.5% or 1%, were a subject of research. Dacarbazine is an alkylating cytostatic prodrug and a triazene compound whose mechanism of action is due to the ability of its major metabolite, diazomethane, to form covalent alkyl bonds with the molecules containing electron centers (e.g., –SH groups). Since dacarbazine is structurally similar to purine bases, it acts as an antimetabolite, inhibiting DNA synthesis in tumor cells. Dacarbazine 0.5% or 1% was dissolved in distilled water. Thereafter, a mixture of oligo-urethane diisocyanates and the UP-606/2 polymerization accelerator were added, and these components were mixed. Foamed composites were dried in an oven at 25 degrees C until adhesion failure occurred. A series of PUUF samples containing dacarbazine at a weight percent of 0.5% or 1% were obtained. Dacarbazine-free PUUF samples were used as controls. The PUUFs obtained in this way appeared as fine porous sponges (Fig. 1).
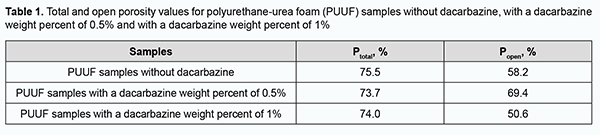
Porosity study of PUUF samples Total porosity (a measure of all the void space of porous material) and open or apparent porosity (a measure of the interconnected void space which communicates with the surface of the test specimen) were determined for the obtained PUUF samples. The equation for total porosity (Ptotal) by percent is: Ptotal = (1-ρa/ρ) х 100, where ρa=m⁄V is an average density (a material weight per unit volume); ρ is a true density without pores (for the adhesive composition based on a mixture of olygourethan diisocyanates, ρ = 1.14). The equation for open porosity (Popen) is: Popen= ((m2-m1 )/V)∙(1/ρw )∙100 where m2 – m1 is the difference between the saturated and dry weight sample; V is the volume of the material; ρw is water density. Study of dynamics of dacarbazine release from PUUF samples in vitro In vitro study of drug release dynamics is an important part of the study of polymer materials with biological activity. The profiles of dacarbazine release from PUUF samples were assessed spectrophotometrically. PUUF samples containing dacarbazine at a weight percent of 0.5% or 1% were used to assess dacarbazine release profiles. Three dacarbazine-containing PUUF samples from each series and a dacarbazine-free PUUF control sample were used for statistical significance assessment. With this in mind, 20 ml distilled water was added to each sample placed into a stoppered box, and this mixture was incubated at 37±1°C. Extracts were regularly spectrophotometrically evaluated and compared with the controls for optical density. Histological studies Soft tissue cellular responses and biocompatibility of the developed composites were assessed through the implantation of the latter in Wistar rats weighting 200-230 g. Animals were divided into three groups of 12 animals. Dacarbazine-free PUUF samples were implanted in animals of group 1; PUUF samples containing dacarbazine at a weight percent of 0.5%, in animals of group 2, and PUUF samples containing dacarbazine at a weight percent of 1%, in animals of group 3. All manipulations on experimental animals were performed under anesthesia in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series [20]. Surgical procedures on animals were done under aseptic conditions. The operative field was prepared, and a polymer sample (sized 10.0 х 5.0 х 5.0 mm) was subcutaneously implanted in the interscapular space without suture fixation, to exclude the influence of suturing on wound healing. This space is slightly mobile and a space in the body which the animal cannot reach. At days 7, 14, 30 and 90 postimplantation, rats were euthanized by an overdose of chloroform. Implanted polymer samples were collected with surrounding connective tissue and fixed in 10% buffered formalin solution. This was followed by processing, embedding in paraffin, sectioning to 10-15 μm, mounting and hematoxylin and eosin staining in a routine manner [21]. Histological sections were examined by light microscopy. Results Porosity study of PUUF samples The total porosity of obtained PUUF samples ranged slightly from 73.7% to 75.5%, whereas the open porosity depended both on the presence and weight percent of dacarbazine (Table 1). Of note that the open porosity substantially decreased for the PUUF samples containing dacarbazine at a weight percent of 1%, but not for the samples containing dacarbazine at a weight percent of 0.5% or dacarbazine-free samples. In addition, in the PUUF samples containing dacarbazine at a weight percent of 1%, the number of open pores was almost equal to the number of closed pores, which may contribute to gradual tissue fluid leakage into the samples and fibrin precipitation, providing the basis for the formation of tissue structure. Therefore, the number of open pores may be important early after implantation, enabling the impact not only on reliable implant placement, but also on subsequent implant survival.
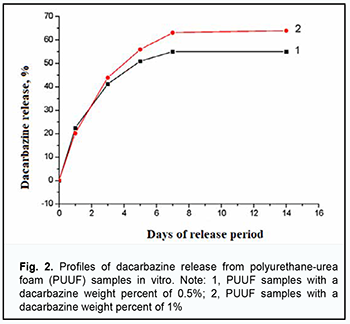
In vitro study of dynamics of dacarbazine release from PUUF samples The ultraviolet absorption spectra of the examined extracts before and after polymer sample exposure were identical to the spectrum of dacarbazine. The absorption spectrum of dacarbazine had a maximum at 329 ± 2 nm. At incubation days 1 to 3, there was no significant difference in dacarbazine release between the PUUF samples containing dacarbazine at a weight percent of 1% and those containing dacarbazine at a weight percent of 0.5%. At days 5, 7 and 14, an increase in dacarbazine release was higher in the former samples than in the latter samples (64% versus 55% or less). Fig. 2 shows the profiles of dacarbazine release from PUUF samples.
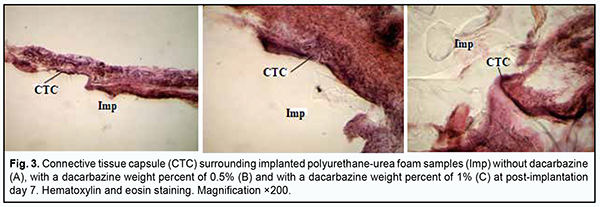
Histological studies The behavior response and the external state of animals as well as the postoperative field were assessed during the experiment. The epithelial response at the operative site was visually assessed daily, and it was found that the wound healed within three days without signs of inflammatory response. Morphological signs of degenerative changes, tumors, or tissue necrosis were observed neither in the early nor in the late postoperative period. Implanted materials were readily palpated through the skin throughout the experiment. There was no aggression or behavioral changes in experimental animals after implantation of polymer samples. During material harvesting, we macroscopically observed connective tissue capsules around all implanted samples, with no signs of severe inflammation. The connective tissue surrounding the implant facilitated reliable implant placement, with no implant displacement or migration in the subcutaneous space throughout the experiment. No morphologically apparent changes (like exudates, necrosis, or suppuration) were observed at the site of PUUF sample implantation. A tight network of blood vessels surrounding an implanted PUUF sample was found, with an increase in thickness and density of some connective tissue capsules at the late post-implantation time points. At day 7 after implantation of a dacarbazine-free PUUF sample, an inmature connective tissue capsule was observed around the sample, with the capsule comprised of polymorphonuclear neutrophils, monocytes, macrophages and young fibroblasts (Fig. 3а). Elongated and spindle-shaped fibroblasts entrapped within mature collagen bundles were observed at some small sites of the capsule. Numerous dilated and engorged newly-formed mid-size and large-size blood vessels were observed in the field of vision, and some of them had signs of stasis and thrombosis.
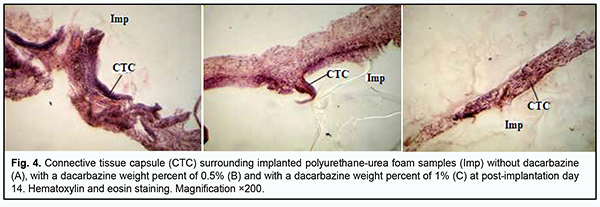
At day 7 after implantation of a PUUF sample containing dacarbazine at a weight percent of 0.5%, implanted material was seen separated from the surrounding tissues by a wide cuff of lymphocytes and a thick connective tissue capsule. Round cells (primarily, neutrophils and lymphocytes) were observed in the capsule tissue, and apparent monocyte/macrophage infiltration was seen (Fig. 3b). In addition, young fibroblastic elements without definite signs of maturity and low differentiated cells were seen nearby at this time point. Spindle-shaped fibroblasts entrapped within mature collagen bundles were observed at some small sites of the capsule. There were numerous blood vessels, some of them were dilated and engorged, and some vessels had signs of stasis and thrombosis. At day 7 after implantation of a PUUF sample containing dacarbazine at a weight percent of 1%, implanted material was seen separated from the surrounding tissues by a wide cuff of lymphocytes and a thick connective tissue capsule. Neutrophils, macrophages and young fibroblasts were the most common cell types (Fig. 3c). At some sites of the capsule surrounding the implant, the development of proliferative process due to the active production of extracellular matrix components and bundles of mature collagen fibrils by fibroblast cells was observed. The blood vessels around the implant were few in number and characterized by normal microcirculatory processes. At day 14 after implantation of a dacarbazine-free PUUF sample, there was an increase in connective tissue capsule thickness. In addition, typically, the number of macrophages increased compared with the previous time point, indicating the activation of phagocytic processes. Moreover, local marked neutrophil and lymphocyte infiltration and young fibroblasts were observed. At some sites, the capsule was more mature than at other sites, and elongated and spindle-shaped fibroblasts entrapped within mature collagen bundles were observed. At this time point, marked protrusion and ingrowth of connective tissue into the microporous polymer sample were typically seen (Fig. 4a). Blood vessels were still numerous, and some of them were engorged and had signs of stasis and thrombosis.
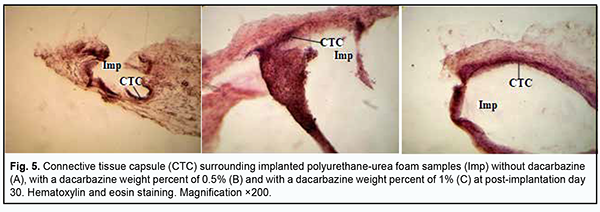
At day 14 after surgery, the connective tissue capsule surrounding a PUUF sample containing dacarbazine at a weight percent of 0.5% appeared thinner and better shaped than that surrounding a dacarbazine-free PUUF sample. The inner layer of the capsule was characterized by marked round-cell response (primarily, neutrophil and macrophage response). In addition, mild lymphocyte infiltration was seen at some locations of the inner layer. The outer layer of the connective-tissue capsule consisted of mature waved collagen bundles separated by spindle-shaped fibroblasts (Fig. 4b). The number of blood vessels substantially decreased due to their reduction compared to the previous time point, with normal microcirculatory processes in these vessels. At day 14 after implantation of a PUUF sample containing dacarbazine at a weight percent of 1%, there was a variation in the degree of maturity of the connective-tissue capsule surrounding the sample. At some locations, the capsule consisted of mature waved collagen bundles separated by spindle-shaped fibroblasts (Fig. 4c), whereas other locations showed marked neutrophil infiltration with increased monocyte/macrophage response. There was a moderate number of blood vessels, with normal microcirculatory processes in these vessels. At day 30, there was an increase in the degree of maturation of the connective-tissue capsule surrounding a dacarbazine-free PUUF sample due to the active production of extracellular matrix components and mature collagen bundles by fibroblast cells (Fig. 5a). The cellular composition of the capsule was heterogeneous throughout its length, with apparent leukocyte and macrophage infiltration at some locations, with other sites showing young fibroblasts and spindle-shaped fibroblasts entrapped within mature collagen bundles. There was a moderate number of blood vessels, with normal microcirculatory processes in these vessels.
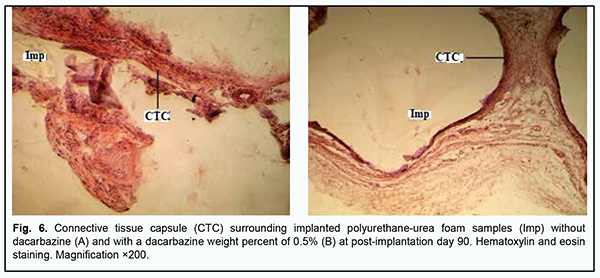
At day 30 after implantation of a PUUF sample containing dacarbazine at a weight percent of 0.5%, there was an increase in inflammatory cellular response in the porous structure of implanted samples (Fig. 5b), with a significant increase in the numbers of neutrophils, lymphocytes and macrophages. There was no significant increase in the number of blood vessels compared to the previous time point, with most vessels showing normal microcirculatory processes. There were isolated vessels with a dilated lumen showing stasis and thrombosis. At day 30 after implantation of a PUUF sample containing dacarbazine at a weight percent of 1%, there was a rather thin connective tissue capsule surrounding the implant, with the cellular composition of the capsule including fibroblasts with increased synthesis of collagen and extracellular matrix. The number of macrophages was still high at this time point (Fig. 5c). There were a moderate number of blood vessels, with normal microcirculatory processes in these vessels, and isolated vessels with a dilated lumen showing leukocyte margination and no stasis or thrombosis. At day 90 after implantation of a dacarbazine-free PUUF sample, the connective-tissue capsule surrounding the sample showed a high degree of maturity throughout its length. The capsule consisted of mature waved collagen bundles separated by spindle-shaped fibroblasts and showed an increase in capsule density due to increased synthetic activity of fibroblasts. At some sites of the capsule, there were small accumulations of macrophages with increased phagocytic activity (Fig. 6a).
At day 90 after implantation of a PUUF sample containing dacarbazine at a weight percent of 0.5%, the connective-tissue capsule showed a rather high degree of maturity. Inflammatory cells were of low number, and the most common cells were spindle-shaped fibroblasts entrapped within mature collagen bundles. At some sites of the capsule, there were small accumulations of macrophages with increased phagocytic activity, which may be explained by a reaction of the surrounding tissues to the foreign body, and may indicate an activation of implant biodegradation process. Connective tissue strands showed increased branching and ingrowth into polymer samples (Fig. 6b). There was a moderate number of blood vessels, with normal microcirculatory processes in these vessels. At day 90 after implantation of a PUUF sample containing dacarbazine at a weight percent of 1%, the cellular responses were similar to those seen at this time point after implantation of a sample containing dacarbazine at a weight percent of 0.5%, with the connective-tissue capsule surrounding the implant showing a high degree of maturity and organization throughout its length. Inflammatory cells were of low number, and the most common cells were spindle-shaped fibroblasts entrapped within mature collagen bundles. At some sites of the capsule, there were small accumulations of macrophages with increased phagocytic activity. Connective tissue strands showed increased branching and ingrowth into polymer samples. There was a moderate number of blood vessels, with normal microcirculatory processes in these vessels. Therefore, cellular responses of the tissues of experimental animals to the implantation of PUUF samples containing either no dacarbazine or dacarbazine at a weight percent of 0.5% or 1% did not result in the pathological processes in the surrounding tissues. At early time points after surgery, cell adhesion and migration began at the internal surface of the pores due to the porous structure of implanted PUUF samples, the structure acting as a scaffold for the formation of connective tissue strands. As early as 7 days after surgery, histology found the implanted sample to be separated from the surrounding tissue through the formation of a wide protective leukocyte wall and the development of the connective tissue capsule. The formation of the connective tissue capsule around implanted PUUF samples varying in the composition and porosity was a completely natural, biologically determined and well predicted process. Early postoperative cellular responses were more reactive and intensive around PUUF samples containing dacarbazine than around PUUF samples containing no dacarbazine. Marked neutrophil and lymphocyte infiltration was characteristic dor these responses, and intensive monocyte and macrophage reaction was observed. In addition, active angiogenesis process was seen around all implant samples, contributing to the release of metabolic products from the focus of inflammation. It was found that dacarbazine was released from the implanted sample to the surrounding tissues over a long period of time, manifesting biological activity and increasing the duration of inflammation at the implant placement site due to highly reactive cellular responses. After day 30, we observed the normalization of cellular responses around almost all implanted cells, with the formation of a mature connective-tissue capsule with fibroblasts showing active synthesis of collagen fibrils and other extracellular matrix components. Porous structure of PUUF samples facilitated gradual ingrowth of cells from the surrounding tissues into implanted samples, causing the development of neutrophil and leukocyte infiltration in their structure and marked macrophage reaction at all post-implantation time points. Macrophages were actively involved in implementation of the complementary protective mechanisms. Discussion Medicine currently requires developing synthetic polymer materials and introducing them into medical practice [10]. Synthetic polymer implantable materials can completely meet medical material requirements and are characterized by a wide range of basic characteristics contributing to their advanced biocompatibility and biointergration at the site of their application [6, 7]. The spatial porosity of implantable materials facilitates ingrowth of the surrounding tissues of the living organism, which improves implant survival with a reliable implant placement, and, in some cases, the elastic structure of these systems may contribute to reduced pressure on, and damage to, these tissues [18]. We believe that drug immobilization onto polyurethane foam carriers is an important task aimed at obtaining biocompatible polymer materials for restorative and reconstructive surgical procedures. The basic characteristics of these materials will allow not only improving their performance at the site of application, but also reducing the toxicity of the immobilized drug and prolonging drug release. Since developing these implantable materials is promising, we believed it reasonable to select the optimal component ratio for the synthesis of a series of polyurethane-urea foams with immobilized dacarbazine. The total porosity of obtained PUUF samples ranged from 73.7% to 75.5%, and the open porosity ranged from 50.6% to 69.4%. The percent of small open pores in PUUF samples containing dacarbazine at a weight percent of 1% was approximately 50%, and could substantially impact the morphological features of cellular reactions and subsequent cell proliferation through intensive connective tissue ingrowth into a porous matrix. The profiles of dacarbazine release from the PUUF samples containing dacarbazine at a weight percent of 1% were different from those of dacarbazine release from the PUUF samples containing dacarbazine at a weight percent of 0.5%. Dacarbazine release from the former PUUF samples was more complete and longer, with a percent release of 64% over 14 days. We reviewed soft tissue cellular responses to implantation of the developed implant samples in experimental animals and demonstrated that early after implantation, these samples were completely separated from the surrounding tissue through the formation of a connective tissue capsule around them. Until post-implantation day 30, we observed formation of a mature connective tissue capsule comprising mostly fibroblasts that synthesized collagen and other extracellular matrix components. It was demonstrated that the presence of dacarbazine in composite materials resulted in long-term cellular inflammation at the implant placement site, which is likely associated with the biological action of the drug. One may benefit from this pattern of cellular responses when using this composition of the composite material in restorative and reconstructive surgical procedures after excision of a malignant tumor. Conclusion First, the total porosity of obtained PUUF samples ranged from 73.7% to 75.5%, and the open porosity ranged from 50.6% to 69.4%. The percent of small open pores in PUUF samples containing dacarbazine at a weight percent of 1% was approximately 50%, and could substantially impact the morphological features of cellular reactions and subsequent cell proliferation through intensive connective tissue ingrowth into a porous matrix. Second, the profiles of dacarbazine release from the PUUF samples containing dacarbazine at a weight percent of 1% were different from those of dacarbazine release from the PUUF samples containing dacarbazine at a weight percent of 0.5%. Dacarbazine release from the former PUUF samples was more complete and longer, with a percent release of 64% over 14 days. Third, early after implantation, the implanted samples were completely separated from the surrounding tissue through the formation of a connective tissue capsule around them. Until post-implantation day 30, we observed formation of a mature connective tissue capsule comprising mostly fibroblasts that synthesized collagen and other extracellular matrix components. Finally, the presence of dacarbazine in composite materials resulted in long-term cellular inflammation at the implant placement site, which is likely associated with the biological action of the drug. One may benefit from this pattern of cellular responses when using this composition of the composite material in restorative and reconstructive surgical procedures after excision of a malignant tumor. References
1.Grusha OV, Grusha IaO. [Five hundred orbital plastic repairs: analysis of complications]. Vestn Oftalmol. 2006 Jan-Feb;122(1):22-4. Russian.
2.Gundorova RA, Neroiev VV, Kashnikov VV. [Eye injuries]. Moscow: GEOTAR-Media; 2009. Russian.
3.Denisenko VD, Galatenko NA, Rozhnova RA, Nechaieva LIu. [Medical dacarbazine-containing polyurethane composites based on polyurethane-urea foams. In: Proceedings of the International Conference on Theoretical and Experimental Aspects of Current Chemistry and Materials]. Dnipro, May 20, 2022. p.32-5. Ukrainian.
4.Krasnovid TA. [Ocular trauma under present conditions. Providing urgent care in Ukraine]. Proceedings of the Conference of Ophthalmologists of Chernihiv, Kyiv, Poltava, Sumy and Cherkasy regions of Ukraine. Chernihiv; September 12-13, 2013. pp. 40-4. Russian.
5.Tselomudryi AI, Venger GE, Rizvaniuk AV, Pogorelyi DN. [Surgical rehabilitation of military servicemen with combat eye injuries under current conditions. In: Proceedings of the Filatov Memorial Lectures 2016 Conference]. May 19-20, 2016; Odesa. p.95. Ukrainian.
6.Sevast’ianov VI, Kirpichnikov MP, editors. [Biocompatible materials: a tutorial]. Moscow: Meditsinskoie informatsionnoie agenstvo; 2011. Russian.
7.Uvarova IV, Maksymenko VB. [Bio‐compatible Materials for Medical Products. Educational Manual. Biomedical Engineering Department. National Technical University of Ukraine “KPI”)]. Kyiv: KIM, 2013. Ukrainian.
8.Gerasimenko VG, editor. [Biotechnology: a textbook]. Kyiv: INKOS; 2006. Ukrainian.
9.Rudenchyk TV, Rozhnova RA, Galatenko NA, Nechaieva LIu. [Impact of a biological medium model on the structure and characteristics of levamisole-containing composites and drug release patterns]. Voprosy Khimii i Khimicheskoi Tekhnologii. 2018;5:140-8. Ukrainian.
10.Galatenko NA, Rozhnova RA. [Biologically active polymeric materials for medicine]. Kyiv: Naukova Dumka; 2013. Russian.
11.Panarin IeF, Lavrov NA, Solovskii MV, Shalova LI. [Polymers as carriers of biologically active compounds]. Moscow: Professiia; 2014. Russian.
12.Rudenchyk TV, Rozhnova RA, Galatenko NA. [Medical unsaturated ethers and their derivatives]. Polimernyi zhurnal. 2018;40(4):216-29. Ukrainian.
13.Babker A, Sotnik S, Lyashenko V. Polymeric Materials in Medicine. Sch J Appl Med Sci. 2018;6:148–153. doi: 10.21276/sjams.2018.6.1.33.
14.Kozlova GA, Rozhnova RA, Nechaieva LIu, Galatenko NA. [Impact of a medium model on the structure and characteristics of doxorubicin-containing and with composites based on polyurethane with isocyanurate fragments]. Polimernyi zhurnal. 2021; 43(1):54-63. Ukrainian.
15.Kuliesh DV, Nechaieva LIu, Galatenko NA. [Dynamics of release of methyluracil, a synthetic immunostimulator, from implantable polymer composites]. Dopovidi Natsionalnoi akademii nauk Ukrainy. 2015;3:122-6. Ukrainian.
16.Rudenchyk TV, Rozhnova RA, Galatenko NA, et al. [Developing composites based on oligooxipropylene fumarate, triethylene glycol dimethacrylate and styrole with prolonged levamisole release]. Dopovidi Natsionalnoi akademiyi nauk Ukrayiny. 2016;11:78-86. Ukrainian.
17.Stashenko KV, Rudenchyk TV, Galatenko NA, Rozhnova RA. [Synthesis and properties of composites based on based on polyurethane urea with fragments of a copolymer of polyvinyl butyral (vinyl acetate with vinyl alcohol) with vinyl alcohol and lysozyme]. Pytannia khimii ta khimichnoi tekhnolohii. 2020;1:71-9. Ukrainian.
18.Khodorenko VN, Yasenchuk YuF, Gyunter VE. [Biocompatible materials and implants with shape memory. In: Gyunter VE, editor. Biocompatible porous penetrable materials]. Northampton: STT; Tomsk: STT. p.9-24. Russian.
19.Colnik M, Hrncic MK, Skerget M, Knez Z. Biodegradable polymers, current trends of research and their applications, a review. Chem Ind Chem Eng. Q. 2020;26:401–418.
20.European convention for the protection of vertebrate animals used for experimental and other scientific purposes. Council of Europe, Strasbourg; 1986.
21.Bagrii MM, Dibrova VA, editors. [Methods of morphological research].Vinnytsia: Nova knyha. 2016. Ukrainian.
Disclosures
Corresponding Author: Maletskii Anatolii, maletskiy@filatov.com.ua
Author Contribution:
Galatenko N. A.: concept development, design, data collection, data analysis and interpretation, manuscript preparation/writing/review;
Rozhnova R. A.: concept development, design, data analysis and interpretation, manuscript preparation/writing/review;
Kuliesh D. V.: concept development, design, data collection and research, data analysis and interpretation, manuscript preparation/writing/review;
Denisenko V. D.: conducting research, analysis and interpretation of data, preparation of manuscript;
Maletskyy A. P.: concept development, design, data analysis and interpretation, manuscript preparation/writing/review;
Bigun N.M.: data analysis and interpretation, manuscript preparation/writing/review.
All authors analyzed the results and agreed on the final version of the manuscript.
Conflict of Interests.The authors declare that there is no conflict of interest that could influence their opinion regarding the subject matter or materials described and discussed in this manuscript.
Sources of Support. The article is a part of the research work on the topic "Formation of polymers of different spatial structure and polymer-based compositions for immobilization of biologically active compounds and medicinal substances", registration number #0114U007099.
Disclaimer: The considerations presented in the article are exclusively the author's and do not represent the official position of the institutions.
The research was conducted on animals. The experiment was performed in accordance with the "European Convention for the Protection of Vertebrate Animals Used for Experimental or Other Scientific Purposes" (Strasbourg, 1986).
Abbreviations: PUUF – polyurethane-urea foam; DC – dacarbazine; CTC – connective tissue capsule
|