J.ophthalmol.(Ukraine).2022;4:28-32.
|
http://doi.org/10.31288/oftalmolzh202242832 Received: 13.07.2022; Accepted: 10.08.2022; Published on-line: 24.08.2022 Features of visual disturbances in patients with newly diagnosed acromegalia K. S. Iegorova 1, L. V. Zadoianyi 1, M. O. Guk 1, O. Ie. Skobska 1, M. L. Solovei 1, 2, O. G. Chernenko 1, B. B. Guda 2 1 SI "Romodanov Neurosurgery Institute of the National Academy of Medical Sciences of Ukraine"; Kyiv (Ukraine) 2 SI «V.P. Komisarenko Institute of Endocrinology and Metabolism»; Kyiv (Ukraine) TO CITE THIS ARTICLE: Iegorova KS, Zadoianyi LV, Guk MO, Skobska OIe, Solovei ML, Chernenko OG, Guda BB. Features of visual disturbances in patients with newly diagnosed acromegalia. J.ophthalmol.(Ukraine).2022;4:28-32. http://doi.org/10.31288/oftalmolzh202242832 Background: Acromegaly is a complex neuroendocrinologic disorder associated with hypersecretion of growth hormone (GH) and insulin-like growth factor (IGF-1) in individuals with completed physiological growth. The cause is, most commonly, an autonomously functioning pituitary adenoma (PA) secreting GH only or GH in combination with prolactin. Cuevas-Ramos and colleagues identified three structural and functional acromegaly types distinguished by clinical course, direction of tumor extension, tumor aggressiveness, expression profile of somatotroph surface receptors and potential responsiveness to treatment. Purpose: To review the features of visual disturbances in patients with newly diagnosed acromegaly. Material and Methods: We retrospectively reviewed the medical records of 174 patients with newly diagnosed acromegaly who underwent endoscopic transnasal transsphenoidal surgery for PA at the Transsphenoidal Neurosurgery Department of the Romodanov Institute during 2018 through 2011. In all patients, the diagnosis of PA was confirmed by a comprehensive morphological study. Of the 174 patients, 11.5% (20 patients; 40 eyes) had visual disturbances (reduced visual acuity and/or visual field defects) and were included in the visual disturbance group. Patients underwent clinical and neurological, endocrinological and eye examination and neuroimaging studies. Results: Visual disturbances were found preoperatively in 20 (11.5%) of the 174 consecutive patients with PA secreting GH only. Of the 20 cases, 17 (85%) were classified as type 3, and 3 (15%), as type 2 according to the structural and functional acromegaly classification of Cuevas-Ramos et al. Suprasellar extension of the pituitary adenoma towards the optic nerve-chiasm complex and compression of the crossed optic nerve fibers caused symmetric chiasmal syndrome in 11 (55%) and primary descending optic atrophy in 14 (70%) patients. After treatment, the numbers of eyes with severe and very severe visual acuity loss reduced by 5% and 7.5%, respectively, and a reduction in visual field defects was observed in 12.5% of eyes. Conclusion: We believe that the application of the structural and functional acromegaly classification by Cuevas-Ramos and colleagues would be useful not only for assessing whether a multimodal approach (comprising neurosurgery, medical therapy and radiotherapy) is required, but also for predicting the risk of developing visual disturbances in a particular case of acromegaly. Keywords: pituitary adenoma, acromegaly, structural and functional classification, compressive optic neuropathy, endoscopic transnasal surgery
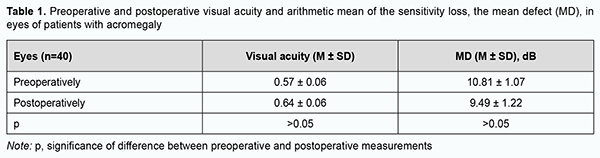
Introduction Acromegaly is a complex neuroendocrinologic disorder associated with hypersecretion of growth hormone (GH) and insulin-like growth factor (IGF-1) in individuals with completed physiological growth. The cause is, most commonly, an autonomously functioning pituitary adenoma (PA) secreting GH only or GH in combination with prolactin. It has been reported that autonomously functioning PAs secreting GH only account for 25% of all autonomously functioning PAs and for 2-17% of all PAs [1, 2, 3, 4, 5]. Acromegaly is characterized by pathological and disproportionate growth of bones, soft tissues and internal organs, and various metabolic abnormalities (diabetes mellitus, lipid abnormalities, cholelitiasis, hyperthyroidism, splanchnomegaly, respiratory complications, cardiovascular complications, hypertension, impaired calcium metabolism, and peripheral neuropathy) [6, 7, 8, 9, 10]. A multidisciplinary team approach is recommended for effective management of acromegaly, with the first-line treatment being transnasal transsphenoidal surgery for PA [2, 3, 4]. However, recent multicenter epidemiological studies on acromegaly have demonstrated that surgery was the only treatment required for clinical and laboratory remission in no more than 5 percent of patients that underwent surgery for acromagalia [11]. Visual disturbances are the major clinical features in many somatotropic PAs and exert mass effects on the optic nerve/chiasm complex, with a reduction in visual acuity in 38%-68.5% and visual field abnormalities in 39.8%-70% of patients [12, 13, 14, 15, 16]. The presence of visual disturbances is generally considered a major indication for urgent surgery [2, 5, 17]. Since there are numerous pathomorphological variants of GH-secreting pituitary adenomas differing in clinical picture and resistance to treatment, a strong interest has emerged to identify the clinical and biological prognostic factors in acromegaly in order to optimize the treatment strategy for patients with the disease. Cuevas-Ramos and colleagues [5, 18] aimed to rigorously classify an acromegaly patient cohort defined by clinical, radiological, histopathological, and outcome characteristics, and developed a structural and functional acromegaly classification. They identified three structural and functional acromegaly types distinguished by clinical course, tumor aggressiveness and treatment responsiveness, expression profile of somatotroph surface receptors and markers of cell senescence, and disease outcomes. Type1 comprised older patients with the longest follow-up and most favorable outcomes, characterized by densely granulated, non-aggressive microadenomas and macroadenomas. Tumors extended to the sphenoid sinus more frequently than the suprasellar region (a concave MRI shape), and, therefore, visual disturbances were rarely seen in these patients. Mass effects were usually not observed in these tumors. GH and IGF-1 levels at diagnosis were moderately increased, which was reflected by mild somatic changes. A higher proportion of type 1 patients have Ki-67 index <3%, indicating lower proliferative activity of tumor cells. Expression of somatostatin receptor 2 (SSTR2) and responsiveness to treatment with somatostatin analogs were higher in type 1. Type 2 comprised noninvasive, densely or sparsely granulated macroadenomas with no invasive features. Densely granulated adenomas in this group responded less effectively to treatments than type 1 patients. Although these tumors seem less aggressive, type 2 patients have higher GH and IGF-1 levels at diagnosis, and required more treatments than did type 1 patients. In addition, these tumors may be identified with a flat MRI shape and characteristic invasion of the cavernous sinus space. Abundance of SSTR2 and p21 immunoreactivity was lower than in type 1, which was compatible with clinically manifested acromegaly. Type 3 comprised more aggressive and sparsely granulated macroadenomas. These tumors extend to both the sphenoid sinus and suprasellar regions with commonly encountered significant optic chiasm compression, and can be identified on MRI as a “peanut” or round shape. The lower expression of alpha-subunit, p21, and SSTR2 may also reflect reversion to a less differentiated somatotroph cell, without cycle arrest and more aggressive tumor growth. High tumor aggressiveness correlated with very low p21 expression and decreased SSTR2 expression likely led to attenuated treatment responsiveness [5, 18]. In recent years, attention has been given to different clinical scenarios of acromegaly; patients with acromegaly may be conventionally divided into those with aggressive and those with mild disease. The natural history of acromegaly is characterized by chronic progressive disability and a shortened life span. Approximately 50% of untreated acromegalic patients die by the age of 50 [19]. Studies estimate acromegaly to have a total prevalence of 2.8 to 13.7 cases per 100,000 people [7]. Studies on the analysis of visual disturbances in acromegaly are rare [19, 20, 21]. The purpose of the study was to review the features of visual disturbances in patients with newly diagnosed acromegaly. Material and Methods We retrospectively reviewed the medical records of 174 patients with newly diagnosed acromegaly who underwent endoscopic transnasal transsphenoidal surgery for PA at the Transsphenoidal Neurosurgery Department of the Romodanov Institute during 2018 through 2011. In all patients, the diagnosis of PA was confirmed by a comprehensive morphological study. Of the 174 patients, 11.5% (20 patients; 40 eyes) had visual disturbances (reduced visual acuity and/or visual field defects) and were included in the analysis. The group included 17 women (85%) and 3 men (15%) with a mean age of 46.2 ± 11.6 years. Patients with advanced tumor growth, history of radiotherapy or radiosurgery, or ocular comorbidities were excluded. Patients underwent clinical and neurological, endocrinological and eye examination. Imaging studies (pituitary MRI with contrast), laboratory studies (measurements of serum GH, IGF-1, prolactin, cortisol, thyroid stimulating hormone and FT4 levels), and a comprehensive study of tumor tissue morphology (including light microscopy, immunohistochemistry, and expressions of SSTR2 and SSTR5) were performed. A neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, kinetic and static perimetry, and direct and indirect ophthalmoscopy. Preoperative examination was performed at day 1 or 2 after admission, and postoperative examination, at day 5 to 7 after surgery. Patients were divided into groups on the basis of visual acuity: group 1 (normal vision, a BCVA of 1.0 or better), group 2 (mild visual disturbance, a BCVA of 0.7-0.9), group 3 (moderate visual disturbance, a BCVA of 0.4-0.6), group 4 (severe visual disturbance, a BCVA of 0.1-0.3), group 5 (very severe visual disturbance, a BCVA worse than 0.1), and group 6 (blindness, a BCVA of 0). Static automated perimetry (SAP) threshold and suprathreshold tests were performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany). Visual field loss severity was classified as “no visual field loss” (Grade 0; normal visual field), mild visual field loss (Grade 1; MD, –2 dB to –4 dB), moderate visual field loss (Grade 2; MD, –4 dB to –12 dB), severe visual field loss (Grade 3; MD, –12 dB to –20 dB), and very severe visual field loss (Grade 4; MD, worse than –20 dB). A chiasmal syndrome was considered symmetric if both eyes had the same grade of visual acuity or visual field loss. In addition, a chiasmal syndrome was considered asymmetric if the difference between eyes in grade of visual acuity or visual field loss severity was 1, and it was considered markedly asymmetric if the difference was 2 or greater. A two-fold improvement (for postoperative VA worse than 0.1) or improvement by 0.2 or more (for postoperative VA better than 0.1) in VA with at least a 15 percent visual field expansion, reduction in scotoma size, or any improvement in MD was considered a positive change in visual function. The study adhered to the tenets of the Declaration of Helsinki, and was approved by the Bioethics Committee of the Romodanov Neurosurgery Institute. Written informed consent was signed by all patients. Results are presented as the mean and standard error of mean (M ± SD). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ≤ 0.05 was assumed. Results Visual disturbances were found preoperatively in 20 (11.5%) of the 174 consecutive patients with PA secreting GH only. Based on the MRI features and findings of the subsequent comprehensive morphological study of pituitary adenoma tissue, 17 cases (85%) were classified as type 3, and 3 cases (15%), as type 2 according to the structural and functional acromegaly classification of Cuevas-Ramos et al. The duration of visual disturbances was several days to three years, with a gradual decrease in visual function. Of note that 3 patients (15%) had no visual complaints, but exhibited changes in visual acuity and/or visual field defects on initial eye examination. A PA with mild suprasellar extension only was observed in 8 patients (40%), with suprasellar and parasellar extension, in 5 patients (25%). In addition, a giant PA with several pathways of extrasellar extension was observed in 7 patients (35%). Two patients (10%) exhibited ocular motility disorders due to lesions in the intracavernous portions of cranial nerves III, IV and VI. Preoperatively, BCVA was 1.0 in both eyes in 4 patients (20%), and 16 patients (80%) exhibited a reduction in visual acuity, either unilateral or bilateral. The BCVA was 1.0 in 12 eyes (30%); 0.7-0.9 in 8 eyes (20%); 0.4-0.6 in 5 eyes (12.5%); 0.1-0.3 in 7 (17.5%) eyes, and <0.1 in 7 eyes (17.5%). In addition, one eye (2.5%) had no vision. No visual field defects were found in 2 eyes (5%). Visual field defects were found in 38 eyes (95%). Temporal hemianopia (either complete or partial) only was the commonest field defect (10 eyes; 25%), followed by temporal hemianopia with central scotoma (8 eyes; 20%), central scotoma only (8 eyes; 20%); homonymous hemianopia (6 eyes; 15%), and residual visual field with a loss of central vision (3 eyes; 7.5%). In addition, visual field was not measurable due to extremely low visual function in 3 eyes (7.5%). In the eyes included in the analysis, moderate visual field loss was most common (23 eyes; 57.5%), followed by severe visual field loss (8 eyes; 20%), and mean preoperative MD was 10.81±1.07 dB (Table 1).
Symmetric chiasmal syndrome was most common (11 eyes; 55%), followed by markedly asymmetric (7 eyes; 35%) and asymmetric (2 eyes; 10%). Ophthalmoscopy found primary descending optic atrophy (OA) in 14 (70%) patients. Of these, 10 patients (20 eyes) exhibited bilateral OA, and 4 patients (4 eyes), unilateral OA. Postoperatively, BCVA was 1.0 in 13 eyes (32.5%); 0.7-0.9 in 10 (25%) eyes; 0.4-0.6 in 7 (17.5%) eyes; 0.1-0.3 in 5 eyes (12.5%), and <0.1 in 4 eyes (10%). In addition, one eye (2.5%) had no vision. BCVA maintained at 1.0 in 10 (25%) eyes, restored to 1.0 in 3 (7.5%) eyes, improved in 13 (32.5%) eyes, did not change in 8 (20%) eyes, and worsened in 6 (15%) eyes. Numbers of eyes with severe visual acuity loss reduced by 5%, and with very severe visual acuity loss, by 7.5%. Postoperatively, 7 (17.5%) eyes exhibited no visual field defects, and 33 (82.5%) eyes, visual field defects. In addition, temporal hemianopia (either complete or partial) only was the commonest field defect (10 eyes; 25%), followed by temporal hemianopia with central scotoma (9 eyes; 22.5%), central scotoma only (5 eyes; 12.5%); homonymous hemianopia (4 eyes; 10%) and residual visual field with a loss of central vision (3 eyes; 7.5%). In addition, visual field was not measurable due to extremely low visual function in 2 eyes (7.5%). In addition, visual field remained normal in 2 (5%) eyes, improved in 17 (42.5%) eyes, did not change in 11 (27.5%) eyes, restored to normal in 5 (12.5%) eyes, and worsened in 5 (12.5%) eyes. Visual field defects reduced in size in 12.5% of eyes. Postoperatively, BCVA and MD improved, but not statistically significantly (p > 0.05). Discussion The incidence of visual disturbances in a large cohort of patients with newly diagnosed acromegaly was rather low (11.5%), which is in agreement with the findings of the leading neuroendocrinological centers. This reflects not only early diagnosis of acromegaly and use of neurosurgical treatment at early stages of the disease, but also, and more importantly, phenotypic heterogeneity of sporadic acromegaly, with a spectrum of large groups (types 1 and 2 by Cuevas-Ramos et al) in which visual disturbances rarely occur [3, 5, 18]. In our newly diagnosed acromegaly patients with visual disturbances, symmetric chiasmal syndrome was most common, indicating an injury to crossed optic nerve fibers, which is associated with the predominance of pituitary lesions with suprasellar symmetric extension towards the optic nerve-chiasm complex, and is reflected by the predominance of temporal visual field defects. This is in agreement with the findings of others [19, 20]. This predominance is in agreement with biological characteristics of PA in type 3 acromegaly by Cuevas-Ramos et al [18]. Rivoal and colleagues [20] reviewed 307 cases of acromegaly seen from 1951 through 1996 at a single referral center. Abnormal visual field, either at the time of diagnosis or during follow-up, decreased from 27% of patients between 1951 and 1975 to 15.4% of patients between 1976 and 1996, when modern neuroimaging techniques became available. Visual field defects in macroadenomas associated with acromegaty range in incidence from 5 to 40% in several series reported between 1970 and 1987 [19]. Although modern neuroimaging and laboratory techniques do enable establishing the diagnosis of acromegaly and reduce the incidence of acromegaly patients with visual disturbances, this is related mostly to type 3 acromegaly, where there is a potential for suprasellar extension of the pituitary adenoma. Therefore, we believe that the application of the structural and functional acromegaly classification by Cuevas-Ramos and colleagues would be useful not only for assessing whether a multimodal approach (comprising neurosurgery, medical therapy and radiotherapy) is required, but also for predicting the risk of developing visual disturbances in a particular case of acromegaly. In the current study, postoperatively, BCVA and MD improved, but not statistically significantly (p > 0.05), possibly due to a long follow-up and lack of attention to visual functions in patients with autonomously functioning PA.
References 1.Kovacs K, Horvath E. Tumors of the pituitary gland. Atlas of Tumor Pathology, series 2, vol. 21. Washington: Armed Forces Institute of Pathology. 1986. 2.Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass J, et al. A Consensus Statement on acromegaly therapeutic outcomes. Nature reviews. Endocrinology. 2018; 14(9), 552–561. 3.Fleseriu M, Biller B, Freda PU, Gadelha MR, Giustina A, Katznelson L, et al. A Pituitary Society update to acromegaly management guidelines. Pituitary. 2021; 24(1), 1–13. 4.Giustina A. Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, et al. “Multidisciplinary management of acromegaly: A consensus.” Rev Endocr Metab Disord. 2020: 21(4), 667-678. 5.Taweesomboonyat C, Oearsakul T. Prognostic Factors of Acromegalic Patients with Growth Hormone-Secreting Pituitary Adenoma After Transsphenoidal Surgery. World Neurosurg. 2021;146: e1360-e1366. 6.Ritchie CM, Atkinson AB, Kennedy AL, Lyons AR, Gordon DS, Fannin T, Hadden DR. Ascertainment and natural history of treated acromegaly in Northern Ireland. Ulster Med J. 1990; 59(1): 55-62. 7.Lavrentaki A, Paluzzi A, Wass J, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20(1):4-9. 8.Daly A, Rixhon M, Adam C, Dempegioti A, Tichomirowa M, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769-75. 9.Holdaway I M, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008; 159(2):89-95. 10.Khizhnyak O, Barabash N, Mikityuk M, Nikolaev R, Gogitidze T. Clinical and hormonal features of acromegaly in patients from a Ukrainian neuroendocrinology centre. Probl Endocrine Pathol. 2018; 65(3):67-74. 11.Trainer P. J. ACROSTUDY: an overview. Horm Res. 2007; 68 (5); 68–69. 12.Abouaf L, Vighetto A, Lebas M. Neuro-ophthalmologic exploration in non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015; 76(3):210-9. 13.Foroozan R. Chiasmal syndromes. Curr. Opin. Ophthalmol. 2003; 14(6):325-31. 14.Kitthaweesin K, Ployprasith C. Ocular manifestations of suprasellar tumors. J Med Assoc Thai. 2008; 91(5):711-5. 15.Wadud SA, Ahmed S, Choudhury N, Chowdhury D. Evaluation of ophthalmic manifestations in patients with intracranial tumours. Mymensingh Med J. 2014; 23(2):268-71. 16.Sefi-Yurdakul N. Visual findings as primary manifestations in patients with intracranial tumors. Int J Ophthalmol. 2015; 8(4):800-3. DOI: 10.3980/j.issn.2222-3959.2015.04.28. eCollection 2015. 17.Cardinal T, Rutkowski M, Micko A, Shiroishi M, Jason Liu C, Wrobel B, et al.Impact of tumor characteristics and pre- and postoperative hormone levels on hormonal remission following endoscopic transsphenoidal surgery in patients with acromegaly. Neurosurg Focus. 2020; 48(6):E10. 18.Cuevas-Ramos D, Carmichael J, Cooper O, Bonert V, Gertych A, Mamelak A, Melmed S. A structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015;100(1):122-131. 19.Hennessey JV, Jackson IM. Clinical features and differential diagnosis of pituitary tumours with emphasis on acromegaly. Baillieres Clin Endocrinol Metab. 1995;9(2):271-314. 20.Rivoal O, Brézin AP, Feldman-Billard S, Luton JP. Goldmann perimetry in acromegaly: a survey of 307 cases from 1951 through 1996. Ophthalmology. 2000; 107(5):991-7. 21.Lee A. G. Acromegaly and junctional visual field loss. Ophthalmology. 2001; 108(5), 832–833.
Disclosures Received 13.07.2022 Accepted 10.08.2022 Corresponding Author: Iegorova Kateryna, iegorova_katya@ukr.net. Author Contribution: K.S. Iegorova: Conceptualization, Formal analysis, Writing-original draft; L.V. Zadoianyi: Writing-review & editing; M.O. Guk: Methodology, Project administration, Writing-review & editing; O.Ie. Skobska: Formal analysis, Writing-review & editing; M.L. Solovei: Software, Writing-review & editing; O.G. Chernenko: Writing-original draft; B.B. Guda: Writing-review & editing. All authors reviewed the results and approved the final version of the manuscript. Conflict of Interest: The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or material discussed in this manuscript. Sources of Support: None. Disclaimer: The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions. The study involved human subjects, adhered to the tenets of the Declaration of Helsinki, and was approved by the Ethics Committee. Informed consent was obtained from all patients. This study was not conducted on animals. Abbreviations: pituitary adenoma (PA), optic nerve atrophy (ONA), insulin-like growth factor (IGF-1), magnetic resonance imaging (MRI), growth hormone analogue (GHA), mean defect (MD)
|