J.ophthalmol.(Ukraine).2022;4:23-27.
|
http://doi.org/10.31288/oftalmolzh202242327 Received: 20.05.2022; Accepted: 25.07.2022; Published on-line: 24.08.2022 High-dose intravitreal chemotherapy in the treatment of high-risk retinoblastoma N. F. Bobrova, T. A. Sorochynska, S. A. Tronina, T. V. Romanova, O. Yu. Bratishko SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine)
TO CITE THIS ARTICLE: Bobrova NF, Sorochynska TA, Tronina SA, Romanova TV, Bratishko OYu. High-dose intravitreal chemotherapy in the treatment of high-risk retinoblastoma. J.ophthalmol.(Ukraine).2022;4:23-27. http://doi.org/10.31288/oftalmolzh202242327 Background: Intravitreal chemotherapy (IVitC; intravitreal cytostatic injections) is the most promising field of multiagent chemotherapy for retinoblastoma, and, when combined with systemic multiagent chemotherapy, enables maximum effects on the tumor. Purpose: To improve the method of primary combination multiagent chemotherapy by increasing the melphalan dose and shortening the interval between the first and last injections in IVitC for high-risk retinoblastoma. Material and Methods: As per the improved methodology, melphalan was intravitreally injected at a dose of 20 or 30 µg in 0.1 ml, depending on the type of tumor and type of vitreous seeds, with the interval between intravitreal injections shortened to 10-11 days. The treatment method proposed was used in 8 eyes with a large high-risk tumor (7 eyes with a T3 tumor and one eye with a T2 tumor) in 7 patients aged 42 ± 4.1 months (range, 2 to 60 months). Conclusion: We have developed a method of treatment for high-risk T3 retinoblastomas with or without vitreous seeds which involves local chemotherapy at an increased dose of 20 µg or 30 µg every 10 to 14 days, in the presence of combination multiagent chemotherapy incorporating systemic multiagent chemotherapy, chemoreduction, every three weeks. Our clinical studies demonstrated that the use of the method proposed resulted in tumor size reduction, tumor density increase and tumor calcification, with necrotic changes in vitreous seeds in eyes poorly responsive to the standard intravitreal dose of 10 µg melphalan in 0.1 ml diluent. The major indications for an increase in intravitreal melphalan dose to 20 µg are large T2 or T3 tumors located in the macular or juxtpapillary retina, with loss of tumor capsule integrity, dust vitreous seeds or cloud vitreous seeds. In addition, in the absence of parental consent for enucleation, an intravitreal melphalan dose can be increased to 30 µg as an alternative to enucleation for large T3 tumors with tumor capsule rupture, release of tumor fragments into the vitreous, spherical vitreous seeds, and blindness with no potential for vision restoration. Keywords: retinoblastoma, intravitreal chemotherapy, combination chemotherapy, children
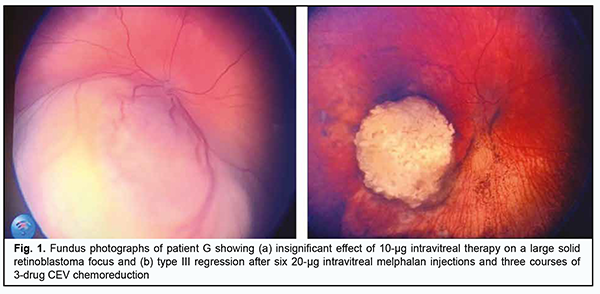
Introduction Intravitreal chemotherapy (IVitC; intravitreal cytostatic injections) is the most promising field of multiagent chemotherapy for retinoblastoma, and, when combined with systemic multiagent chemotherapy, enables maximum effects on the tumor. The method of primary combination multiagent chemotherapy [1, 2] has been developed at the Department of Pediatric Eye Disorders, the Filatov institute. It involves the use of both intravitreal and systemic pathways and enables the highest level of chemotherapeutic agent after its direct injection into the ocular cavity, with a potential reduction in the total toxic load on the child’s body [3, 4]. Special attention should be given to the selection and optimal mode of administration and dosing of the cytostatic, aiming to damage cancer cells without causing too much harm to normal ocular tissues. Melphalan is a well-recognized cytostatic agent for IVitC, but the optimal time interval between injections and dose for injections have not been finally determined until now. The purpose of the study was to improve the method of primary combination multiagent chemotherapy by increasing the melphalan dose and shortening the interval between injections in IVitC for high-risk retinoblastoma. Material and Methods A method for treating an advanced or recurrent retinoblastoma (AJCC T3 tumor) has been developed at the Department of Pediatric Eye Disorders, the Filatov institute. The method involves a combination multiagent therapy comprising systemic chemoreduction and intravitreal Alkeran at an increased dose of 20 µg or 30 µg in 0.1 ml, and with the interval between intravitreal injection time points shortened to 10-14 days depending on tumor stage, size, and proliferation and types of vitreous seeds [5]. The treatment method proposed was used in eight eyes with a large high-risk tumor (7 eyes with a T3 tumor and one eye with a T2 tumor) in seven patients aged 42 ± 4.1 months (range, 2 to 60 months). Of these seven children, three had a bilateral retinoblastoma (3 eyes with a T3 tumor and one eye with a T2 tumor), and four (4 eyes) had a unilateral retinoblastoma (a T3 tumor). A multifocal retinoblastoma was found in 3 patients (3 eyes), and sphere-type and/or cloud-type seeds were found in the vitreous in 4 patients (4 eyes), including 3 patients (3 eyes) with injury-related seeds. All the children (8 eyes) received IVitC in one to six courses, as a portion of combination multiagent chemotherapy. A 20-µg melphalan dose was employed in six eyes. In two of these six eyes, this dose was used after a 10-µg melphalan dose was found to be of inadequate efficacy (Fig. 1a). A 30-µg melphalan dose was employed in two eyes. In one of these two eyes, this dose was used after a 20-µg melphalan dose was found to be of inadequate efficacy. The mean interval between intravitreal injections was 10.7 ± 1.2 days (range, 10 to 14 days). The mean number of intravitreal injections per eye was 5.1 ± 1.6 (range, 3 to 10 days) for a 20-µg melphalan dose, and 8 ± 3 (range, 4 to 12 days) for a 30-µg melphalan dose. Results After treatment, tumor base area decreased by a mean value of 5.9 ± 1.7 mm to 5.7 ± 1.35 mm, and tumor prominence decreased to 1.57 ± 1.07 mm (Fig. 1b). No complications were observed during or early after surgery.
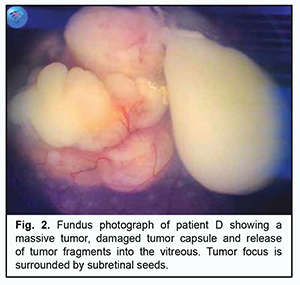
Consolidation therapy included additional laser photocoagulation of tumor foci in 6 children (8 eyes), intra-arterial chemotherapy with alkeran plus topotecan in one child, and brachytherapy in two children. The follow-up period ranged from 4 months to 5 years. Late complications included retinal degenerative changes of various severities. Visual acuity in the preserved eye ranged from light perception to 0.13. The treatment resulted in preservation of all the study eyes and tumor control with tumor size reduction, tumor consolidation and resolution of tumor seeds. Eight children (9 eyes) achieved complete tumor regression. In addition, apparent regression of the tumor and vitreous seeds is observed in one eye, and the child is still under treatment. Example case A four-year-old girl presented to us after being referred due to a complaint of a white pupil in her eye. An eye examination under general anesthesia revealed a large tumor focus in the lower outer quadrant of the fundus. The focus spread over the posterior pole and was completely covered by an amorphous portion of the tumor which was swaying above the focus due to rupture of the tumor capsule and massive spread of the tumor into the vitreous cavity (Fig. 2). Ultrasound scan of the right eye showed a mural mass at the optic disc, with a prominence of 7 mm and a base as large as 17 mm, and with 4.5-mm x 9.5-mm encapsulated low-echogenic a suspended mass above the former mass. The patient was diagnosed with a stage T3b retinoblastoma in the right eye on the basis of clinical and imaging data.
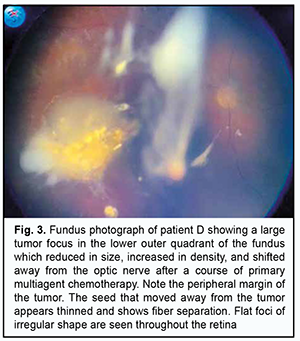
Taking into account examination findings, еnucleation of the affected eye was recommended, but parental consent for the еnucleation was not given. As a last resort to save the eye, intravitreal melphalan at a dose of 30 µg was used in the right eye. This resulted in a significant reduction in tumor size, with a prominence of 3.5 mm and tumor base of 5.0 х 5.5 mm, as assessed by ultrasound imaging (Fig. 3).
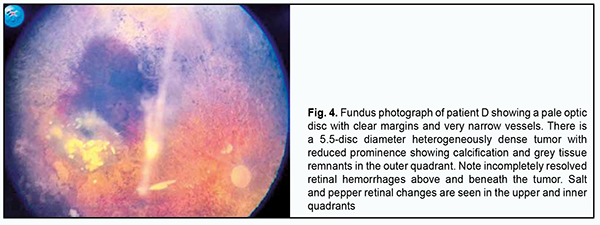
In ten days, the patient received the second intravitreal melphalan injection at the same dose as previously, with adjunctive intravenous CEV therapy. Totally, the eye was treated with 19 intravitreal melphalan injections at a dose of 30 µg during 10 days and four courses of CEV, with a significant tumor regression (Fig. 4). Ultrasound imaging showed a reduction in prominence to 0.82 mm and tumor basal diameter to 2.88 mm, complete calcification, seed resolution and a reduction in seeding class (from sphere to cloud or dust).
At the 12-month follow-up, there was Type 1 tumor regression with complete calcification and vitreous seed regression, and with vitreous opacities in the form of strands undergoing gradual resolution and reduction in size. Discussion Foreign studies have investigated the effects of different melphalan doses on the outcome of treatment for retinoblastoma. Kaneko and Suzuki [6] evaluated 8 μg/0.1 mL and later 20 to 30 μg/0.1 mL of intravitreal melphalan, acknowledging improved control using the latter dose. Sixty-eight percent of the eyes achieved complete vitreous seed remission, but recurrence occurred in 19 % of these eyes after 10.0 ± 4.9 months. In addition, 47 and 27 % of the eyes without primary macular tumors retained visual acuity of >0.5 and >1.0, respectively. Distant metastasis or intracranial invasion occurred in 11 patients, all of whom had high-risk pathological factors for metastasis such as optic nerve invasion, but refused to receive adjuvant chemotherapy [7]. In a study by Munier and colleagues [8], 23 heavily pretreated patients presenting vitreous seeding were treated with an intravitreal injection of melphalan (20-30 µg in 0.1 ml) once a week. The authors reported that retinal toxicity as well as the rate of ocular complications was low. Globe retention was achieved in 87% of cases, and there were no cases of retinoblastoma recurrence over a mean 22 months’ follow-up [8]. Ghassemi and Shields [9] reported on the outcomes of 12 patients with retinoblastoma and refractory or recurrent vitreous tumor seeding managed with intravitreal melphalan. Of those 8 eyes that received 8- to 10-μg doses of melphalan, vitreous seed control was found in 43% of cases at long-term follow-up, and there were minor ocular toxicities. For the 4 eyes that received a 50-μg dose, control was achieved in 100% cases at both short-term and long-term follow-up, but serious adverse effects of hypotony/phthisis bulbi were noted. A more recent study by Shields and colleagues [10] included 11 consecutive eyes of 11 patients with viable persistent or recurrent vitreous seeds following treatment of retinoblastoma. All eyes received intravitreal melphalan injection (20-30 µg) once a month. There were a total of 55 injections, with a mean number per patient of 5. Globe salvage was attained in all cases (100%) after 2 to 6 (median, 4) intravitreal chemotherapy injections. Ghassemi and Khodabande [11] conduct IViC for patients with the following indications: recurrent or residual vitreous seedings after systemic or IAC and monocular patients with any type of vitreous seedings after completion or during the systemic chemotherapy or IAC. They inject the IViC drugs on a biweekly schedule until the vitreous seeds disappear or crystallize. If any viable seeds are visible in any part of the vitreous cavity, despite some crystallization, the injections are continued. Previously, we have conducted an experimental study [12, 13] to assess the impact of different (5-µg to 40- µg) doses of melphalan given in different modes of intravitreal injection on the rabbit retina. The study demonstrated that the severity of changes post intravitreal melphalan injection in the rabbit retina depended on injection dose and schedule. Electron microscopy showed destructive changes in the retinal pigment epithelial (RPE) cells, photoreceptor cells and Müller cells (MC). Morphological changes in RPE and photoreceptor cells were noted after 5-µg intravitreal melphalan injection. The severity and depth of lesions in the retina increased with an increase in injection dose and frequency: compared to re-injection of 5-µg intravitreal melphalan, re-injection of 10-µg intravitreal melphalan caused more substantial changes in RPE and photoreceptor cells, with maintenance of hypertrophic Müller cell processes. One or two 20-µg intravitreal melphalan injections caused severe changes in the structure of the retina, leading to retinal gliosis and necrosis. 30-µg or 40-µg intravitreal melphalan injections caused damage to not only all retinal structures, but also to the choriocapillary layer, with a replacement of destroyed retinal cells by hypertrophic Müller cell processes. Studies [14-16] reported good clinical treatment outcomes of eye-salvage treatment with 10-µg intravitreal melphalan injections for retinoblastoma. The efficacy of primary multiagent chemotherapy with 10-µg intravitreal melphalan injections was 100% for stage T1 retinoblastoma, 95.2% for stage T2 retinoblastoma, and only 60-71.4% for stage T3 retinoblastoma [3]. An increase in the efficacy of eye-salvage treatment for large T3 tumors and vitreous seeds can be achieved only by an increase in the dose of intravitreal cytostatic, because a dose of systemic multiagent chemotherapy should not be increased due to its toxic impact on the child’s body. Therefore, a high melphalan doses of 20 µg or greater causes cytotoxic retinal injury [12] and appear to be deleterious for visual prognosis; however, despite a negative impact of the cytostatic on the retina, a high-dose IViC can be performed for a large posterior-pole tumor that covers the optic disc, affects the macula and thus causes poor primary vision, in an attempt to achieve tumor destruction and eye salvage. The requirement for high melphalan doses in intravitreal chemotherapy is grounded by the cases when eye-salvage therapy is performed as a last resort as an alternative to enucleation for large T3 retinoblastomas. High-risk retinoblastomas with tumor capsule rupture and vitreous and/or subretinal seeds require a more aggressive effect that can be achieved only with an increased dose of intravitreal cytostatic. Conclusion Therefore, we have developed a method of treatment for high-risk T3 retinoblastomas with or without vitreous seeds which involves local chemotherapy at an increased dose of 20 µg or 30 µg every 10 to 14 days, in the presence of combination multiagent chemotherapy incorporating systemic multiagent chemotherapy, chemoreduction, every three weeks. Our clinical studies demonstrated that the use of the method proposed resulted in regressive changes in the tumor, with an increase in tumor density, calcification, and necrotic changes in vitreous seeds in eyes poorly responsive to the standard intravitreal dose of 10 µg melphalan in 0.1 ml diluent. The major indications for an increase in intravitreal melphalan dose to 20 µg are large T2 or T3 tumors located in the macular or juxtpapillary retina, with loss of tumor capsule integrity, dust vitreous seeds or cloud vitreous seeds. In addition, in the absence of parental consent for enucleation, an intravitreal melphalan dose can be increased to 30 µg as an alternative to enucleation for large T3 tumors with tumor capsule rupture, release of tumor fragments into the vitreous, spherical vitreous seeds, and blindness with no potential for vision restoration.
References 1.Bobrova NF, Sorochynska TA, Smaglii DV. [Method of combination treatment for retinoblastoma]. Patent of Ukraine UA 55690. Bulletin No.24/2010 issued on March 12, 2018. Ukrainian. 2.Bobrova NF, Sorochynska TA. [Combination (intravitreal and intravenous) multiagent chemotherapy in eye salvage treatment for retinoblastoma]. Oftalmol Zh. 2011;2: 38-44. Russian. 3.Bobrova NF, Sorochynska TA. [Efficacy of combination multiagent chemotherapy for retinoblastoma]. In: [Proceedings of the National Conference on Current Issues in Ophthalmology]. Odesa, Ivano-Frankivsk. 2019; p.9-11. Russian. 4.Seregard S, Kock B, Trampe E. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995 Feb;79(2):194-5. 5.Bobrova NF, Sorochynska TA, Tronina SA, Bratishko OIu. [Method of treatment for high-risk and recurrent retinoblastoma]. Patent of Ukraine UA 101618. Bulletin No.19 issued on January 4, 2021. Ukrainian. 6.Suzuki S, Kaneko A. Vitreous injection therapy of melphalan for retinoblastoma. In: Proceedings of the 15th Biennial Meeting of the International Society of Ocular Oncology. November 15, 2011; Buenos Aires, Argentina. 7.Suzuki S, Aihara Y, Fujiwara M, et al. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol. 2015 May;59(3):164-72. 8.Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013 Jul;27(3):147-50. 9.Ghassemi F, Shields C. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012 Oct;130(10):1268-71. 10.Shields CL, Manjandavida FP, Arepalli S, et al. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014 Mar;132(3):319-25. 11.Ghassemi F, Khodabande A. Risk definition and management strategies in retinoblastoma: current perspectives. Clin Ophthalmol. 2015 Jun 8;9:985-94. 12.Bobrova NF, Sorochynska TA, Molachaniuk NI, Bratishko OIu. Ultrastructural changes in the rabbit retina after various one-time doses of intravitreal melphalan. J Ophthalmol (Ukraine). 2020; 4(495):50-5. 13.Bobrova NF, Sorochynska TA, Levytskyy IM. Different melphalan dozes intravitreal influence on rabbit’s retina. In: [Abstract Book of Ophthalmic oncology group Meeting]. Moscow, Russia; 2015. p. 51. 14.Bobrova NF, Sorochynska TA. [Local chemotherapy for retinoblastoma]. In: [A collection of papers from East-West Ophthalmology Conference]. Ufa;2011. p.374-6. Russian. 15.Bobrova N, Sorochinskaya T. Current retinoblastoma management with intravitreal chemotherapy application. In: [Abstract Book of Joint Congress of SOE/AAO]. 2011; Geneva, Switzerland. p.146-7. 16.Bobrova NF, Sorochinskaya TA, Levytskyy IM. Intravitreal Chemotherapy – the first line of retinoblastoma eye salvage treatment. In: [Book of Abstracts of the 39th Annual Meeting of European Society]. Germany; 2013. p. 82.
Disclosures Corresponding Author: Tronina Svitlana Alfredivna - filatov.detskoe7@gmail.com Disclaimer: The opinions expressed in this article are those of the authors Source of Support: none Declaration of Conflict of Interest: the authors declare that there is no real or potential conflict of interest that could influence the opinion regarding the subject or materials described and discussed in this manuscript.
|