J.ophthalmol.(Ukraine).2022;4:18-22.
|
http://doi.org/10.31288/oftalmolzh202241822 Received: 27.06.2022; Accepted: 25.07.2022; Published on-line: 24.08.2022 Features of aflibercept plus hyperbaric oxygen therapy for diabetic macular edema V. O. Drozdov, V. M. Sakovych Dnipro State Medical University; Dnipro (Ukraine) TO CITE THIS ARTICLE: Drozdov VO, Sakovych VM. Features of aflibercept plus hyperbaric oxygen therapy for diabetic macular edema. J.ophthalmol.(Ukraine).2022;4:18-22. http://doi.org/10.31288/oftalmolzh202241822
Background: Diabetes mellitus (DM) is a disease characterized by chronic hyperglycemia and a cascade of pathological vascular alterations leading to diabetic retinopathy (DR) and diabetic macular edema (DME). Purpose: To compare clinical characteristics in patients treated with aflibercept only and in those treated with aflibercept plus hyperbaric oxygen (HBO) therapy for DME. Material and Methods: This open-label case-control study involved 91 type 2 diabetic (DM2) patients with non-proliferative DR and DME. Patients of the aflibercept-only group received a monthly intravitreous aflibercept at a dose of 2 mg for 5 months. Patients of the aflibercept-plus-HBO group received a monthly intravitreous aflibercept at a dose of 2 mg for 3 months. In addition, the latter patients received ten HBO sessions in the period between the first and the third injections. All patients underwent a routine general clinical and eye examination. Results: At 3 months and 6 months, patients in the aflibercept-plus-HBO group showed improvements in visual acuity by 28.81% and 7.14%, respectively (р < 0.05), and retinal sensitivity to light by 24.44% and 19.5%, respectively (р < 0.05), with a reduction in the number of patients with DME by 84.56% and 51.13%, respectively (p < 0.05). Conclusion: Intravitreal aflibercept with adjunctive HBO provides improved efficacy of treatment for DME in patients with DM2, with reductions in (a) the number and risk of intravitreal injections, (b) drug load on the patient, and (c) treatment duration. Key words: non-proliferative diabetic retinopathy, diabetic macular edema, aflibercept, hyperbaric oxygen therapy
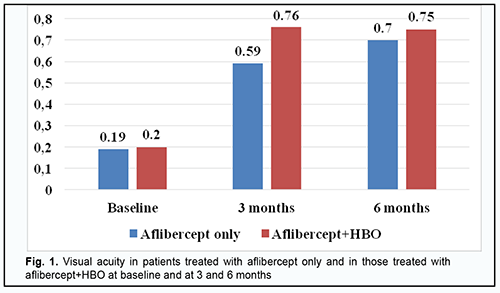
Introduction Diabetes mellitus (DM) is a disease characterized by chronic hyperglycemia caused by a deficiency in insulin action, insulin secretion or both. A cascade of pathological vascular changes results in a wide spectrum of microvascular and macrovascular complications, including diabetic retinopathy (DR) and diabetic macular edema (DME), in patients having long diabetes duration and/or uncontrolled hyperglycemia [1; 2]. DME can occur at any stage of DR [3] and result in loss of visual acuity in as much as 50% of the patients. A framework for understanding the pathophysiology of diabetic macular edema (DME) is the oxygen theory [4, 5]. Prolonged periods of hyperglycemia lead to reduced perfusion of the inner retina and decreased inner retinal oxygen tension. The autoregulatory response of the retinal arterioles causes dilation, which leads to increased hydrostatic pressure in the intraretinal capillaries and venules as specified by Poiseuille’s law [6]. The elevated intravascular pressure experienced by the capillaries may damage them [6, 7]. Concomitantly, the decrease in retinal oxygen tension upregulates the synthesis of VEGF and other permeability factors, which increases microvasculature leakage. According to Starling’s law, increased intravascular pressure and vascular permeability result in a net flow of water, ions, and macromolecules from the intravascular space into the extravascular space. Clinical trials e.g., VISTA and VIVID [8, 9] have demonstrated that the number of anti-VEGF injections required for DME varies widely, with some patients able to achieve remission on several injections, whilst other showing signs of active disease over a year despite monthly intervention [8]. In studies by Wells and colleagues [10], Baker [11] and colleagues and others, median numbers of intravenous anti-VEGF injections required were 7 and 8 per year. Meta-analyses demonstrated that anti-VEGF therapy for DME achieved maximum possible efficacy and there is a requirement for new strategies aimed at other pathophysiological mechanisms of DME formation. Hyperbaric oxygen (HBO) has been proposed as an adjunct to anti-VEGF therapy in the treatment of DME in type 2 diabetic (DM2) patients with non-proliferative diabetic retinopathy (NPDR). Until recently, there have been few studies on the use of HBO for DME in NPDR. However, a study by Maalej and colleagues [12] assessed the effect of HBO on diabetic retinopathy lesions and DME and demonstrated an improvement in visual functions and a decrease in retinal thickness in patients undergoing the treatment for diabetic foot ulcers. Material and Methods This open-label study was conducted at the Department of Ophthalmology of Dnipro State Medical University. The study is a portion of Optimizing the Methods of Diagnosis and Treatment of Retinal and Optic Nerve Diseases in the Presence of Diabetes Mellitus, a research program (Ukrainian State Registration No. 0118U001275; with the research to be conducted from January 2018 through December, 2020) and Improving the Diagnosis and Pathogenetically Grounded Treatment of Ocular Degenerative, Vascular and Inflammatory Disorders, a research program (Ukrainian State Registration No. 0121U111440; with the research to be conducted from January 2021 through December, 2024). Ninety-one type 2 diabetic patients with both NPDR and DME were included in the study after obtaining informed consent. Diabetes mellitus was diagnosed according to the guidelines of the Association of Endocrinologists of Ukraine, European Society of Endocrinology [13] and other normative documents outlined by the Ministry of Health (MOH) of Ukraine. Diabetic retinopathy was diagnosed and patients were treated in accordance with MOH Order No. 117 dated March 15, 2007, On Approval of Protocols for Provision of Eye Care. Diabetic macular edema was graded on clinical examination according to recognized severity scales based on Early Treatment Diabetic Retinopathy Study (ETDRS) criteria [14]. The inclusion criteria were as follows: moderate type 2 diabetic patients with moderate glycemic control, NPDR and DME, and no other retinal and/or optic nerve disorders. The exclusion criteria were as follows: progression to severe diabetes or poor glycemic control, occurrence of uncontrolled arterial hypertension, myocardial infarction or stroke, proliferative DR, media opacity affecting best-corrected visual acuity measurements; refraction errors associated with amblyopia; other retinal and/or optic disc disorders. Mean patient age was 63.75 ± 0.84 years; mean diabetes duration, 12.84 ± 0.63 years; mean systolic blood pressure, 137.99 ± 1.23 mmHg, and mean diastolic blood pressure, 84.62 ± 0.87 mmHg. Study patients were divided into two groups. Patients of the aflibercept-only group (45 patients; 45 eyes) received a monthly intravitreous aflibercept (Eylea; Bayer, Basel, Switzerland) at a dose of 2 mg (50 µL) for 5 months. Patients of the aflibercept-plus-HBO group (46 patients; 46 eyes) received a monthly intravitreous aflibercept (Eylea; Bayer, Basel, Switzerland) at a dose of 2 mg (50 µL) for 3 months. In addition, the latter patients received ten 45-minute sessions of hyperbaric 95-percent medical oxygen at 1.4 atmospheres in a BLKS 301M monoplace chamber in the period between the first and the third injections. Patients received a comprehensive eye examination including visual acuity, refractokeratometry, intraocular pressure, biomicroscopy, ophthalmoscopy, Humphrey automated perimetry (Humphrey Field Analyzer II Carl Zeiss Meditec; Humphrey 10-2 threshold visual field testing), and optical coherence tomography (OCT). Mathematical statistics methods were used for data analysis. Methods of primary statistical analysis were employed for statistical description of the parameters considered in the study. Mean values and standard error of mean values were calculated. Data was assessed for normality using the Kolmogorov-Smirnov test. Distributions of most characteristics were not normal. The Student t test was used when the distribution of the data was normal, whereas the Mann-Whitney when the distribution of the data was not normal. Spearman’s correlation was employed to assess associations between variables. The correlation coefficient was considered significant if P ≤ 0.05. The study followed the ethical standards stated in the Declaration of Helsinki, European Convention on Human Rights and Biomedicine, relevant provisions of the WHO, International Council of Scientific Unions, and International Code of Medical Ethics (1983), and relevant laws of Ukraine. Results In patients of the aflibercept-only group, visual acuity improved from 0.19 ± 0.02 at baseline to 0.59 ± 0.03 (р < 0.001) at 3 months, and to 0.7 ± 0.02 (р < 0.05) at 6 months. In addition, in patients of the aflibercept-plus-HBO group, visual acuity improved from 0.20 ± 0.03 at baseline to 0.76 ± 0.02 (р < 0.001) at 3 months, and maintained at 0.75 ± 0.01 (р < 0.05) at 6 months (Fig. 1).
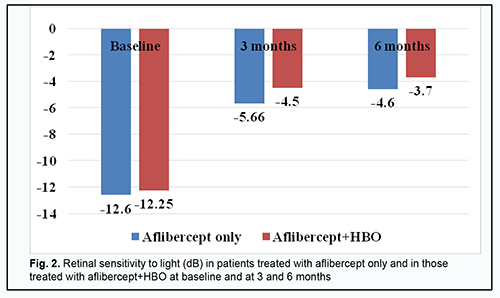
Retinal sensitivity to light (Fig. 2) (a) increased, from -12.60 ± 0.15 dB to -5.66 ± 0.33 Db (р < 0.001), at 3 months, and was -4.6 ± 0.39 Db (р < 0.05) at 6 months, in the aflibercept-only group, and (b) increased, from 12.25 ± 0.12 dB to -4.50 ± 0.27 Db (р < 0.001), at 3 months, and was -3.70 ± 0.2 Db (р < 0.05) at 6 months, in the aflibercept-plus-HBO group.
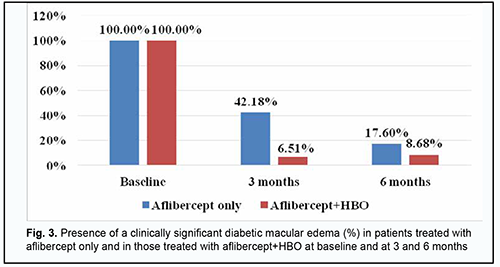
At 3 months and 6 months, DME was seen in 42.18% and 17.6%, respectively, of patients in the aflibercept-only group, and 6.51% and 8.68%, respectively, of patients in the aflibercept-plus-HBO group (Fig. 3).
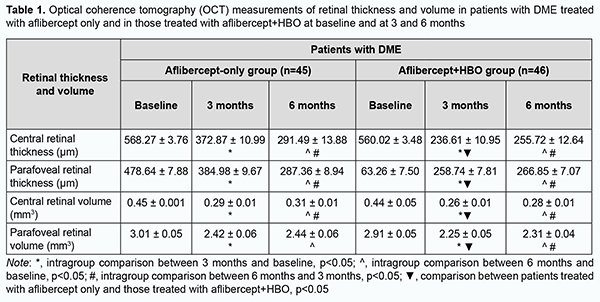
There were statistically significant improvements in OCT-based characteristics with time after initiation of treatment in patients of both groups (Table 1). At 3 and 6 months, in the aflibercept-only group, central retinal thickness decreased by 34.3% and 48.7%, respectively (р < 0.05), and parafoveal thickness, by 19.5% and 39.96% (р < 0.05), respectively. In addition, central retinal volume decreased by 35.5% and 31.1%, respectively (р < 0.05), and parafoveal volume, by 19.6% and 18.9%, respectively. At 3 and 6 months, in the aflibercept-plus-HBO group, central retinal thickness decreased by 57.75% and 54.34%, respectively (р < 0.05), and parafoveal thickness, by 44.15% and 42.4% (р < 0.05), respectively. In addition, central retinal volume decreased by 40.9% and 36.3%, respectively (р < 0.05), and parafoveal volume, by 22.6% and 20.6%, respectively.
Discussion Studies have demonstrated that a framework for understanding the pathophysiology of DME is the oxygen theory [4, 5]. Prolonged periods of hyperglycemia lead to reduced perfusion of the inner retina and decreased inner retinal oxygen tension. The autoregulatory response of the retinal arterioles causes dilation, which leads to increased hydrostatic pressure in the intraretinal capillaries and venules as specified by Poiseuille’s law [6]. The elevated intravascular pressure experienced by the capillaries may damage them [6, 7]. Concomitantly, the decrease in retinal oxygen tension upregulates the synthesis of VEGF and other permeability factors, which increases microvasculature leakage. According to Starling’s law, increased intravascular pressure and vascular permeability result in a net flow of water, ions, and macromolecules from the intravascular space into the extravascular space. HBO has been proposed as an adjunct to anti-VEGF therapy in the treatment of DME in DM2 patients with NPDR. Until recently, there have been few studies on the use of HBO for DME in NPDR. A study by Maalej and colleagues [12] assessed the effect of HBO on diabetic retinopathy lesions and DME and demonstrated an improvement in visual functions and a decrease in retinal thickness in patients undergoing the treatment for diabetic foot ulcers. We hypothesized that the treatment effect of aflibercept for DME in NPDR may be improved by two adjunctive courses of HBO before the first and third injections, in an attempt to improve visual acuity, reduce manifestations of DME and reduce the loading dose. At 3 months, patients in the aflibercept-plus-HBO group showed improvements in visual acuity by 28.81% (р < 0.05) and retinal sensitivity to light by 24.44% (р < 0.05), and reductions in DME thickness by 84.56% (p < 0.05), central and parafoveal retinal thickness by 36.54% (p < 0.05) and 32.79% (p < 0.05), respectively, and central and parafoveal retinal volume by 10.34% (p < 0.05) and 7.02% (p < 0.05), respectively. At 6 months, patients in the aflibercept-plus-HBO group showed improvements in visual acuity by 7.14% (р < 0.05) and retinal sensitivity to light by 19.5% (р < 0.05), and a reduction in the frequency of DME by 51.13% (р < 0.05). These findings suppose that HBO is a beneficial adjunct in the treatment of DME. Conclusion We compared the efficacy of aflibercept-only treatment (a monthly intravitreous aflibercept at a dose of 2 mg (50 µL) for 5 months) and aflibercept-plus-HBO treatment (a monthly intravitreous aflibercept at a dose of 2 mg (50 µL) for 3 months) in patients with DME, and found that either of these treatments can be used. Intravitreal aflibercept with adjunctive HBO provides improved efficacy of treatment for DME in patients with DM2, with reductions in (a) the number and risk of intravitreal aflibercept injections, (b) drug load on the patient, and (c) treatment duration.
References 1.Li J, Welchowski T, Schmid M, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: a systematic review and meta-analysis. Eur J Epidemiol. 2020 Jan;35(1):11-23. 2.Linderman R, Cava J, Salmon A, et al. Visual Acuity and Foveal Structure in Eyes with Fragmented Foveal Avascular Zones. Ophthalmol Retina. 2020 May;4(5):535-44. 3.Holekamp NM. Overview of diabetic macular edema. Am J Manag Care. 2016 Jul;22(10 Suppl):s284-s291. 4.Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006 Jul-Aug;51(4):364-80. 5.Browning D, Stewart M, Lee Ch. Diabetic macular edema. Evidence-based management. Indian J Ophthalmol. 2018 Dec;66(12):1736-1750. 6.Lund-Andersen H. Mechanisms for monitoring changes in retinal status following therapeutic intervention in diabetic retinopathy. Surv Ophthalmol. 2002 Dec;47 Suppl 2:S270-7. 7.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. J Ophthalmic Vis Res. 2014 Jul-Sep; 9(3):362-73. doi: 10.4103/2008-322X.143378. 8.Kodjikian L, Bellocq D, Mathis T. Pharmacological Management of Diabetic Macular Edema in Real-Life Observational Studies. Biomed Res Int. 2018 Aug 28;2018:8289253. 9.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014 Nov;121(11):2247-54. 10.Wells AJ, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015 Mar 26;372(13):1193-203. 11.Baker CW, Glassman AR, Beaulieu WT, et al. DRCR Retina Network. Effect of Initial Management With Aflibercept vs Laser Photocoagulation vs Observation on Vision Loss Among Patients With Diabetic Macular Edema Involving the Center of the Macula and Good Visual Acuity: A Randomized Clinical Trial. JAMA. 2019 May 21;321(19):1880-1894. 12.Maalej A, Khallouli A, Choura R, et al. The effects of hyperbaric oxygen therapy on diabetic retinopathy: A preliminary study. J Fr Ophtalmol. 2020. 2020 Feb;43(2):133-8. 13.Petersmann A, Müller-Wieland D, Müller UA, et al. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2019 Dec;127(S 01):S1-S7. 14.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003 Sep;110(9):1677-82.
Disclosures Corresponding Author: Drozdov Volodymyr, vladimirandco@gmail.com Author Contribution: Drozdov V.O. – data collection and research, data analysis and interpretation, manuscript preparation, writing, reviewing; Sakovich V.M. – concept development, design, manuscript preparation, writing, reviewing. All authors analyzed the results and agreed on the final version of the manuscript. Disclaimer: The opinions expressed and presented in the article are solely the author's and do not represent the official position of the foundation or institution. Sources of support: the present research is a part of the research works performed at the Department of Ophthalmology at the Dnipro State Medical University: "Optimization of diagnosis and treatment methods for pathology of the retina and optic nerve in diabetes mellitus" (state registration number 0118U001275), completion period from 01.2018 to 12.2020 and "Improvement of diagnostics and pathogenetically justified treatment of dystrophic, vascular and inflammatory eye diseases" (state registration number 0121U111440), completion period from 01.2021 to 12.2024. Conflict of Interest: all authors declare no conflict of interests Abbreviations: DM - Diabetes mellitus; DR - diabetic retinopathy; DME - diabetic macular edema; VEGF – vascular endothelial growth factor; HBO - hyperbaric oxygen; DM2 - type 2 diabetic; NPDR - non-proliferative diabetic retinopathy.
|