J.ophthalmol.(Ukraine).2022;2:37-41.
|
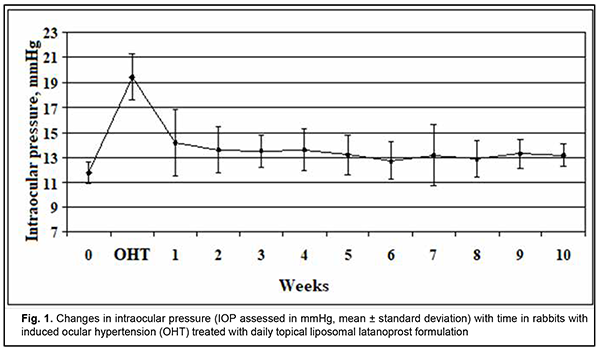
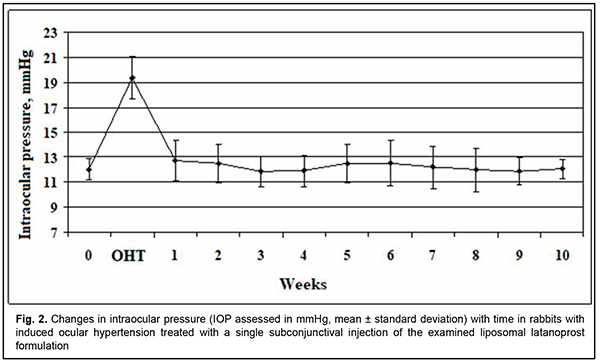
http://doi.org/10.31288/oftalmolzh202223741 Received: 18 November 2021; Published on-line: 30 April 2022 Ocular hypotensive efficacy of a new liposomal latanoprost formulation administered by different routes for experimental ocular hypertension I. M. Mikheytseva 1, G. S. Grygorieva 2, N. V. Pasyechnikova 1, S. G. Kolomiichuk 1, T. I. Siroshtanenko 1, N. F. Konakhovych 2 1 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) 2 SI "Institute of Pharmacology and Toxicology of NAMS of Ukraine"; Kyiv (Ukraine) E-mail: filatovbiochem@ukr.net TO CITE THIS ARTICLE: Mikheytseva IM, Grygorieva GS, Pasyechnikova NV, Kolomiichuk SG, Siroshtanenko TI, Konakhovych NF. Ocular hypotensive efficacy of a new liposomal latanoprost formulation administered by different routes for experimental ocular hypertension. J.ophthalmol.(Ukraine).2022;2:37-41. http://doi.org/10.31288/oftalmolzh202223741 Background: Prostaglandin analogs (e.g., latanoprost) are the first-line therapy for glaucoma. These medications, however, have a short antihypertensive effect due to low penetration of topical drug across the corneal epithelium, which causes the need for their daily application for a long time. Therefore, it is clinically and socially important to develop latanoprost medications with improved efficacy against ocular hypertension (OHT) and with improved patient compliance through the prolonged effect of latanoprost. Purpose: To assess (a) changes in intraocular pressure (IOP) with time and (b) duration of hypotensive effect of a proprietary liposomal latanoprost formulation administered topically or by subconjunctival injection for experimental OHT. Material and Methods: Twenty-one adult Chinchilla rabbits (age, 1 year; weight, 2.5 to 3.0 kg) were divided into three groups: group 1, animals with induced OHT, which was treated with topical liposomal latanoprost (n = 7); group 2, animals with induced OHT, which was treated with a single subconjunctival injection of liposomal latanoprost (n = 7); and group 3, untreated animals with induced OHT, (n = 7). OHT was induced by two 0.1-mL anterior chamber injections of 0.3% carbomer at 10 day intervals. A 0.1-ml subconjunctival injection of liposomal latanoprost formulation was applied immediately after formation of the model of OHT. Topical liposomal latanoprost (one drop per eye) was bilaterally applied at a dose of 1 drop per eye once daily in the evening. Follow-up duration was 10 weeks. IOP was measured in each group before and after OHT modeling. In addition, it was measured after subconjunctival injection of liposomal latanoprost or first application of topical liposomal latanoprost. Thereafter, IOP measurements were performed once a week. Statistica 5.5 (StatSoft, Tulsa, OK, USA) software was applied for statistical analysis. Non-parametric statistical tests for dependent and independent samples were used. Results: We assessed the pharmacological efficacy and duration of hypotensive effect of a proprietary liposomal latanoprost formulation administered topically or by subconjunctival injection for experimental OHT in rabbits. After OHT modeling was performed, there was a persistent increase in IOP, with the IOP values being 51-65% higher than at baseline (р < 0.001). The IOP in animals with OHT treated daily with topical liposomal latanoprost was 30.5% lower than in untreated animals with OHT (р < 0.001). A single subconjunctival injection of the examined liposomal latanoprost formulation resulted in a 36.7% reduction in IOP compared to baseline (р ˂ 0.001), with the effect being as long as 10 weeks. Conclusion: The current study demonstrated a statistically significant hypotensive effect of topical or subconjunctival injection treatment with the examined liposomal latanoprost formulation, with the effect of a single subconjunctival injection of the formulation being as long as 10 weeks. Keywords: liposomal latanoprost formulation, experimental ocular hypertension, intraocular pressure
Introduction Chronic or inadequately treated elevated intraocular pressure (IOP) results in persistent ocular hypertension (OHP) and the development of glaucoma. Strategies for the prevention of exacerbation of elevated IOP include topical IOP lowering medications, laser treatments, and drainage surgery [1, 2]. Beta blockers and prostaglandin analogs are the first-line therapy based on their safety and high efficacy to reduce IOP [3, 4]. The former (a) have a more pronounced hypotensive effect than the latter due to their impact on trabecular and uveoscleral outflow and (b) effectively prevent an abrupt increase and variation in IOP within a day [5, 6]. Latanoprost, a phenyl-substituted prostaglandin F2α analog, is the most commonly used prostaglandin analog for glaucoma, and is hydrolyzed in the eye to the biologically active metabolite, latanoprost acid [7]. Latanoprost ophthalmic solution 0.005% [8] is available for the treatment of glaucoma in different countries under various tradenames such as Xalatan®, Lanoprost, Lanotan, Akistan, etc. These medications, however, have a short antihypertensive effect due to low penetration of topical latanoprost across the corneal epithelium, which causes the need for their daily application for a long time. Most patients of the target group are elderly individuals for whom it is difficult to comply with the only available latanoprost treatment regimen (i.e., topical daily dosing regimen of latanoprost), and the patient’s failure to comply with the treatment plan may result in progression of OHP and glaucoma [9, 10]. Because there is a growing need for prolonging the treatment effect of latanoprost and thus improving latanoprost treatment efficacy and patient compliance, it is important to study various sustained drug delivery systems for latanoprost agents [11]. Liposomal phosholopid preparations of latanoprost are promising for combining prolonged pharmacotherapeutic IOP regulation with various ways of drug delivery to the eye. This is because of physiological affinity among liposomes and positive experience of clinical application of liposomal drug delivery systems, particularly in the treatment of eye disorders [12-14]. Consequently, it is important to study the potential of novel liposomal preparations of latanoprost for optimization of pharmacotherapeutic effect and compliance in patients with elevated IOP. The purpose of this study was to assess (a) changes in IOP with time and (b) duration of hypotensive effect of liposomal latanoprost administered topically or by injection for experimental ocular hypertension. Material and Methods This study aimed to assess the effect of the liposomal latanoprost formulation that was obtained by the method described previously [15] on the eye with experimental OHP. The formulation represents sterile lyophilized powder kept in vials (0.5 mg of latanoprost per vial) at (-18) оC. Independent physical and chemical methods have confirmed the identity and stability of this product as a formulation containing latanoprost in a liposomal matrix on the basis of egg phosphatidylcholine, dipalmitoylphosphatidyl glycerol, cholesterol, and lactose (used as a cryoprotectant). The mean size of the liposome in the emulsion derived from lyophilized formulation was 220 nm, with a highly monodisperse distribution of nanometer dimension structures. Adult Chinchilla rabbits (age, one year; weight, 3.0 to 3.5 kg) were under quarantine for 2 weeks. Thereafter, they were maintained under normal vivarium conditions, fed and watered ad libitum, and used for ophthalmological experiments. All animal experiments were performed in compliance with the General Ethical Principles of Animal Experiments (approved by Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986). Animals were randomized based on general health condition, eye condition, and IOP values. We used a Maklakoff tonometer with a 7.5-g plunger load to perform IOP measurements after topical anesthesia was performed by instillation of 0.5% proparacaine hydrochloride into the conjunctival sac. OHT was induced by two 0.1-mL anterior chamber injections of 0.3% carbomer at 10 day intervals [16,17]. Modeling was performed under general anesthesia with thiopental sodium 10% (1.0 mL/kg, intramuscularly). Ocular hypertension model was formed at day 14 after the first carbomer injection. Twenty-one rabbits were divided in 3 groups: group 1, animals with induced OHT, which was treated with topical liposomal latanoprost (n = 7); group 2, animals with induced OHT, which was treated with a subconjunctival injection of liposomal latanoprost (n = 7); group 3, untreated animals with induced OHT (n = 7). A 0.1-ml subconjunctival injection of liposomal latanoprost was applied immediately after formation of the model of OHT. Topical liposomal latanoprost (one drop per eye) was bilaterally applied at a dose of 1 drop per eye once daily in the evening. The vials with lyophilized latanoprost formulations were allowed to come to room temperature before preparing the injection solution. To prepare the solution for subconjunctival injection, 0.5 ml of sterile water for injection were introduced into a vial with lyophilized latanoprost, and this mixture was shaken for 5 min and used as required immediately. To prepare eye drops, 0.5 ml of sterile water for injection was introduced into a vial, and this mixture was shaken for 5 min. Thereafter, 1.5 ml of the dissolving containing sodium and potassium salts was introduced into a vial, and this mixture was shaken for 5 min at a room temperature. IOP was measured in each group before and after OHT modeling. In addition, IOP was measured after subconjunctival injection of liposomal latanoprost or first application of topical liposomal latanoprost. Thereafter, IOP measurements were performed once a week. Statistica 5.5 (StatSoft, Tulsa, OK, USA) software was used for statistical analysis. Non-parametric statistical tests for dependent samples included Friedman ANOVA and Kendall concordance for multiple comparisons, and Wilcoxon test. Statistical tests for independent samples included Chi squared test and median test for multiple comparisons, and Mann-Whitney test. Results At baseline, in randomized animals, the IOP was within the normal range (11.8-12.4 mmHg), with no significant difference, indicating that the groups of animals for studying the effects of liposomal latanoprost had been formed validly. After ocular hypertension modeling was performed, in each group, there was a persistent increase in IOP to 18.8 to 19.4 mmHg, which was 51-65% higher than at baseline (р< 0.001). In group 1 of animals with ocular hypertension, immediately before the application of topical liposomal latanoprost, the IOP was 64.6% higher than at baseline (р< 0.001). In the presence of application of topical liposomal latanoprost, the IOP was significantly decreased at each IOP measurement time point, but was still statistically significantly higher than at baseline (Fig. 1). The reduction in elevated IOP began at week 1, and increased at week 3. It is noteworthy that at each IOP measurement time point, the IOP in group 1 (animals with ocular hypertension treated with topical liposomal latanoprost) was 30.5% lower than in animals of group 3 (untreated animals with ocular hypertension) (р< 0.001), indicating a significant hypotensive effect of daily topical liposomal latanoprost treatment. In animals of group 2, a single subconjunctival injection of liposomal latanoprost resulted in apparently normalized IOP at each IOP measurement time point, with no significant difference from baseline (Fig. 2). In addition, the IOP in animals with OHT treated with a single subconjunctival injection of liposomal latanoprost was 36.7% lower than in untreated animals with OHT (р< 0.001). In animals of group 3 (the control group with no treatment for OHT), the IOP immediately after OHT modeling was 51.6% higher than at baseline (р< 0.001). In addition, in these animals, the IOP varied in the range of 18.4 mmHg to 19.5 mmHg during the follow-up period. Pairwise comparison of IOP changes both among experimental groups and over time (a) is essential for objective assessment of the hypotensive effect of the liposomal latanoprost formulation, and (b) demonstrated a reduction in IOP as early as week 1, and steady reduction in IOP at week 3 after initiation of topical liposomal latanoprost treatment or after subconjunctival injection of liposomal latanoprost. Chi squared test and median test for multiple comparisons demonstrated a significant difference in IOP among the independent groups was statistically significant over the period of experiment (р< 0.001), which was evidence that liposomal latanoprost is effective in the treatment of OHT. Discussion In the current animal study, the examined liposomal latanoprost formulation caused a percentage reduction in IOP of 31% and 37% for topical medication and subconjunctival injection, respectively, which corresponds to the clinical data (25-37%) reported by others [18] for daily treatment with latanoprost only or the fixed formulation of latanoprost and timololin for patients with ocular hypertension. The principal difference of a single subconjunctival injection of the examined liposomal latanoprost formulation was the treatment effect as long as 10 weeks at least (i.e., the duration of the observation period) with targeted normalization of IOP. Natarajan and colleagues [19-21] reported on prolonged hypotensive effect of a subconjunctival injection of latanoprost-loaded phosphatidylcholine liposomes. Nevertheless, it is not possible to compare their data with the results of the current animal study because Natarajan and colleagues assessed the IOP in normotensive animals only, which we believe is methodologically incorrect. In addition, the predictive ocular therapeutic value of the hypotensive effect in the clinical setting is rather limited, because the proposed liquid-phase form of the preparation does not allow for the stability of the liposomal structure of liposomal latanoprost. The current study demonstrated a statistically significant hypotensive effect of topical or subconjunctival injection treatment with the examined liposomal latanoprost formulation, with the effect of a single subconjunctival injection of the formulation being as long as 10 weeks. Further research on the ocular safety and optimal effect duration for repeat subconjunctival injections will allow to present grounds for considering our new liposomal latanoprost formulation as a potential IOP reducing treatment against OHT and for prevention of the complications thereof, with improved patient compliance.
Conclusion First, our animal study demonstrated that a ten-week daily treatment with a new liposomal latanoprost formulation for induced ocular hypertension had an apparent hypotensive effect, with a 30.5% reduction in IOP compared to baseline (р ˂ 0.001). Second, a single subconjunctival injection of the examined liposomal latanoprost formulation resulted in a 36.7% reduction in IOP compared to baseline (р ˂ 0.001), with the effect being as long as 10 weeks. Finally, subconjunctival injection of the examined liposomal latanoprost formulation is promising in the treatment of patients with elevated IOP, with a potential improvement in pharmacologic efficacy and patient compliance in the treatment of patients with ocular hypertension or glaucoma.
Conflict of Interest Statement. The authors declare no conflict of interest. Funding Support. There are no external sources of funding.
References 1.Zavgorodnia NG, Pasyechnikova NV. [Primary glaucoma: A new look at an old problem]. Zaporizhzhia: Orbita-YUG; 2010. Russian. 2.Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22(1):1-21. 3.Alekseev VN, Levko MA, Al-Gifari M. [Comparative assessment of the efficacy of prostaglandins in combination therapy for primary glaucoma]. Glaucoma. 2009;1:44-48. Russian. 4.Schwartz GF, Tan J, Kotak S. Hyperemia-associated costs of medication changes in glaucoma patients treated initially with prostaglandin analogs. J Ocul Pharmacol Ther. 2009 Dec;25(6):555-61. 5.Andrés-Guerrero V, Vicario-de-la-Torre M, Molina-Martínez IT. Comparison of the in vitro tolerance and in vivo efficacy of traditional timolol maleate eye drops versus new formulations with bioadhesive polymers. Invest Ophthalmol Vis Sci. 2011 Jun 1;52(6):3548-56. 6.Hodzhaiev NS, Chernykh VV, Trunov AN. [Immune and biochemical changes in patients with POAG in the presence of Glauprost F2α prostaglandin analog]. Klinicheskaia oftalmologiia. 2013;13(2):1–4. Russian. 7.Latanoprost. National Library of Medicine. National Center for Biotechnology Information. 2020-08-29. 8.Egorov EA. [Ophthalmology: a Textbook for Medical School Students]. Moscow: Geotar media;2010. Russian. 9.Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007 Oct;144(4):533-40. 10.Stone JL, Robin AL, Novack GD, et al. An objective evaluation of eye drop instillation in patients with glaucoma. Arch Ophthalmol. 2009 Jun;127(6):732-6. 11.Progress in drug and vaccine delivery. Proceedings of International Conferences on Liposome Advances. London-Athens;1990-2018. 12.Ako-Adounvo A, Nagarval R, Oliveira L, et al. Recent patents on ophthalmic nanoformulations and therapeutic implications. Recent Pat Drug Deliv Formul. 2014;8(3):193-201. 13.Grygorieva GS, Krasnopolskii IuM. [Pharmacotherapeutic status of liposomes per se]. Farmakologiia i likarska toksikologiia. 2020;14(4):264-71. Ukrainian. 14.Fathalla D, Fouad E, Soliman G. Latanoprost niosomes as a sustained realease ocular delivery for the management of glaucoma. Drug Dev Ind Pharm. 2020 May;46(5):806-813. 15.Information Bulletin No. 44, based on Pat. of Ukraine №124,724 issued 03.11.2021. Method for treatment of rhegmatogenous retinal detachment complicated by choroidal detachment. Authors: Pylypenko OIa, Grygorieva GS, Krasnopolskii IuM, Konakhovych NF, Mykheitseva IM, Pasyechnikova NV, Prokhorov VV. 16.Kim HG, Park JW. Experimental chronic ocular hypertension by anterior chamber injection of 0.3 % carbomer solution in the rat. Clin Exp Ophthalmol. May-Jun 2013;41(4):404-12. 17.Wang YY. [Experimental study of carbomer glaucoma model in rabbits by injecting different location in anterior chamber]. Ophthalmol. 2009;45:91- 5. Chinese. 18.Diestelhorst M, Larsson LI, European-Canadian Latanoprost Fixed Combination Study. A 12-week, randomized, double-masked, multicenter study of the fixed combination of latanoprost and timolol in the evening versus the individual components. Ophthalmology. 2006;113(1):70–6. 19.US 2013/020216606 А1, Pub Date 22.08.2013. Venkatraman S., Natarajan J.V., Wong T., Yin Chaiang F.B. Liposomal formulation for ocular drug delivery. 20.Natarajan JV, Ang M, Darvitan A, et al. Nanomedicine for glaucoma: liposomes provide sustained release latanoprost in the eye. Int J Nanomedicine. 2012;7:123-31. 21.Natarajan JV, Chattopadhyay S, Ang M, et al. Sustained release of anti-glaucoma drug: Demonstration of efficacy of a liposomal formulation on rabbit eye. Plos One. 2011;6(9):e24513.
|