J.ophthalmol.(Ukraine).2022;2:3-9.
|
http://doi.org/10.31288/oftalmolzh2022239 Received: 02 December 2021; Published on-line: 30 April 2022 Using an improved multilayer amniotic membrane transplantation technique K. V. Sereda, G. I. Drozhzhyna, T. B. Gaidamaka SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: evsereda08@gmail.com TO CITE THIS ARTICLE: Sereda KV, Drozhzhyna GI, Gaidamaka TB. Using an improved multilayer amniotic membrane transplantation technique. J.ophthalmol.(Ukraine).2022;2:3-9. http://doi.org/10.31288/oftalmolzh2022239 Background: Amniotic membrane (AM) is widely used in ophthalmic surgery. There are three major techniques for amniotic membrane transplantation (AMT): ‘onlay’ (‘patch’) technique, ‘inlay’ (‘multilayer transplantation’) and ‘sandwitch’ technique (a combination of the two techniques mentioned before), but there is no universal technique for placing the amnion on the ocular surface for AMT. In conventional multilayer AMT, the membrane is fixed layer by layer with numerous interrupted sutures, which contributes to a severe corneal inflammatory response and the formation of intense corneal opacity. Purpose: To improve the multilayer amniotic membrane transplantation technique. Material and Methods: The method proposed by us consists in forming a two layer or three layer amniotic graft and anchoring it to the surrounding cornea by a row of interrupted 10-0 sutures. Twenty eight patients with corneal ulcers of different causes underwent amniotic membrane transplantation. There were 17 men (60.7%) and 11 women (39.3%). Mean patient age (standard deviation) was 51.3 (0.81) years. Corneal ulcers were categorized based on the etiology as herpetic (7/28, 25%), neurotrophic (10/28, 35.7%), bacterial (3/28, 10.7%), fungous (2/28, 7.2%), autoimmune (3/28, 10.7%) and those caused by rosacea (3/28, 10.7%). Results: After AMT by the proposed technique, there was a reduction in corneal stromal edema at discharge (χ2 = 29.7; p = 0.0005). In addition, corneal stromal infiltration resorbed at 1 month after surgery compared to at discharge (χ2 = 9.16; p = 0.0025). AMT by the proposed technique facilitated the formation of mild focal corneal opacity in 26 patients (92.8%). Conclusion: Our improved AMT technique reduces the number of sutures on the cornea, enables filling the corneal stromal defect and contributes to decreased inflammatory response and early epithelialization of the corneal surface. Keywords: human amniotic membrane, transplantation, corneal ulcer, interrupted sutures
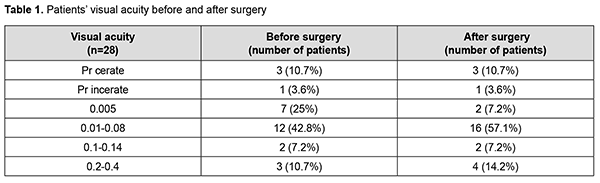
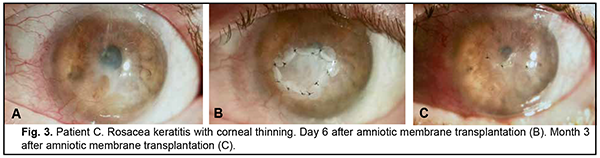
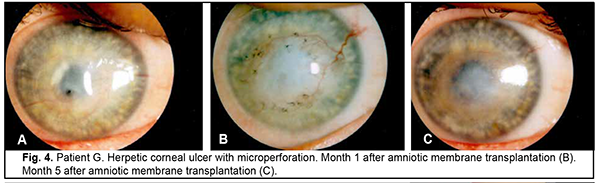
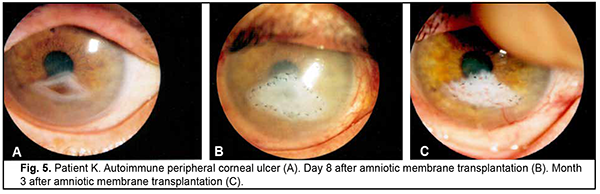
Introduction Severe corneal inflammatory disease and ocular trauma and burns can result in corneal ulceration and perforation, leading to severe conjunctival cicatricial changes, corneal leucoma and substantial visual acuity loss. Local ocular tissues, autografts and donor grafts are commonly used for the treatment of this severe ocular pathology. One of these is the amniotic membrane (AM) which has a variety of unique features and is used in eye surgery [1-3]. Numerous studies demonstrated that AM promotes epithelialization, maintains normal epithelial phenotype, reduces inflammation and scar tissue formation, improves adhesion between tissues, inhibits corneal neovascularization, and has antimicrobial properties [4-6]. The first documented ophthalmological application of the amniotic membrane was in the 1940s [7], but only since 1995 amniotic membrane transplantation (AMT) has been widely used to treat a variety of conditions of the anterior segment of the eye [8-11]. At present, AMT has a role in reconstructive surgery of the ocular surface [12-15] due to its unique properties. The list of indications for the use of AMT in ophthalmology grows year by year [13, 16, 17]. These indications can be divided into such categories as corneal surface reconstruction, conjunctival surface reconstruction, employment as a scaffolding material (in tissue engineering applications), glaucoma, corneomalacia, scleromalacia, etc. There are three major techniques for AMT: ‘onlay’ (‘patch’) technique, ‘inlay’ (‘multilayer transplantation’) and ‘sandwitch’ technique (a combination of the two techniques mentioned before). The ‘onlay’ (‘patch’) technique has been designed to use the AM as a temporary natural patch for the entire surface of the cornea [5]. In the onlay technique, the AM usually dissolves by itself within one to two weeks, as opposed to the multilayer transplantation technique, in which the AM is left over the cornea for life. Indications for the use of this technique vary from acute burns of the eye to acute herpetic keratitis and acute Stevens-Johnson syndrome [6, 18]. The anti-inflammatory properties of the AM are used in this case, but they are efficacious for a limited period of time [19]. Patients with slow epithelialization of the corneal defect may require repeated dressing with the AM [20]. The orientation of the AM plays only a minor role with this surgical procedure, and, most commonly, the AM is secured to the cornea with episcleral or bulbar conjunctival sutures [21]. Dietrich and colleagues [22] have proposed simultaneous AMT in emergency penetrating keratoplasty (PK) as a therapeutic option for corneal destruction, when PK only is accompanied by an increased risk of graft rejection. During the inlay (or multilayer transplantation) procedure, the wound is debrided and the AM is applied with its epithelial/basement membrane side facing upward, then sutured into place with 10-0 nylon sutures. The AM serves as a permanent substitute for the basement membrane; as such, neighboring recipient epithelial cells eventually migrate onto the AM and integrate it into the host cornea. In deep defects, multiple amniotic membranes can be used. Epithelialization of the AM integrates AM into the host tissue. The major indications for this method are persistent epithelial defects, corneal ulceration or to cover defects following excision of conjunctival tumors [23]. The combination (or sandwich) technique is a combination of the two described above and is used mainly in serious disorders of the ocular surface such as deep and extensive corneal ulceration, or in keratoplasty with a high risk of host immune rejection [3]. The main purpose of the onlay is to protect the inlay and promote its epithelialization [8]. This method is favored due to its high success rate (65% to 80%), low rate of recurrence of viral keratitis and persistant epithelial defects (approximately 20% to 35%) [24] and suppression of corneal neovascularization. The purpose of this study was to improve the multilayer amniotic membrane transplantation technique. Material and Methods The proposed surgical technique First, the operative field was cleansed with 0.5% chlorhexidine in alcohol. Epibulbar anesthesia with ophthalmic 0.5% proparacaine hydrochloride (ALCAINE®, SA Alcon-Couvreur NV, Puurs, Belgium) was administered. Dry sulfacyl sodium was administered, if required. A scraper was applied to form a graft bed and remove functionally incompetent epithelial cells. The defect margins were outlined with a marker. A graft of two or more layers was formed from the cryopreserved amniotic membrane by folding the amnion several times onto itself, and the graft was fixed with interrupted 10-0 nylon sutures to the margins of the defect. In all cases, during the surgical procedure, 10-0 nylon interrupted suturing was done in a routine manner, approximately at one half of the corneal thickness. Antiseptic solution was instilled in the conjunctival sac. A soft therapeutic contact lens was placed onto the ocular surface. Dexamethasone and antibiotic injection was administered parabulbarly. Finally, an aseptic monocular patch was placed on the eye. Twenty eight patients with corneal ulcers of different causes underwent amniotic membrane transplantation. There were 17 men (60.7%) and 11 women (39.3%). Mean patient age (SD) was 51.3 (0.81) years. Corneal ulcers were categorized based on the etiology as herpetic (7/28, 25%), neurotrophic (10/28, 35.7%), bacterial (3/28, 10.7%), fungous (2/28, 7.2%), autoimmune (3/28, 10.7%) and those caused by rosacea (3/28, 10.7%). Corneal disease etiology was determined on the basis of history (a recurrent disease course in case of viral keratitis), microbiological study (bacterial of fungal keratitis), corneal sensation assessment (neurotrophic keratitis), presence of general medical condition (autoimmune disease), and/or dermatologist's statement. In most cases (90%), ulcers were of central in location and had a diameter of 4 to 7 mm. Concurrent ocular diseases included myopia (4/28, 14.3%), cataract (11/28, 39.3%), ocular hypertension (7/28, 25%), presence of the graft (3/28, 10.7%), and dry eye disease (6/28, 21.4%). Measures of AMT efficacy included regression of corneal stromal edema, resorption of corneal infiltration, suppression of corneal neovascularization, and epithelialization of corneal surface (assessed by fluorescein staining). Visual acuity was assessed using the Shevalev chart modified by I.A. Viazovskyi and Iu.I. Viazovskyi. Corneal neovascularization (a) was assessed by slit lamp examination and (b) scored by dividing each cornea into 4 quadrants. Primary surgery outcomes included achievement of corneal epithelialization, reduction in corneal inflammation, partial pain relief, and complete corneal epithelialization. Cryopreserved human amniotic membrane (Cryobank of the Institute of Cell Therapy, Kyiv, Ukraine) was utilized in this study. The authors were granted a Certificate for Registration of Copyright for An Improved Multilayer Amniotic Membrane Transplantation Technique No.102,303 issued on February 3, 2021. The results of the improved AMT were assessed at discharge from the in-patient unit, and at 1, 3 and 6 months after surgery. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The level of significance p ≤ 0.05 was assumed. Data are presented as mean (with standard deviation (SD) in parentheses). The chi square test was used for group comparison. Results At admission, the conjunctiva was pink in one patient (3.6%) and moderately hyperemic in 20 patients (71.4%), and mixed conjunctival injection was seen in 7 patients (25%). Corneal stromal edema was apparent in 22 patients (78.5%), moderate in 4 patients (14.3%) and diffuse in 2 patients (7.2%). Punctuate infiltration of the cornea was seen in 11 patients (39.3%); diffuse corneal infiltration, in 6 patients, no corneal infiltration, in 11 patients (39.3%). Vascularization of the limbus was seen in 11 patients (39.3%), vascularization of two corneal quadrants, in 13 patients (46.4%), total corneal vascularization, in 1 patient (3.6%), and no corneal vascularization, in 3 patients (10.7%). The data on visual acuity at admission is presented in Table 1. At admission, visual acuity was 0.005 in 7 patients (25%), 0.01 to 0.08 in 12 patients (42.8%), 0.1 to 0.14 in 2 patients (7.2%), and 0.2 to 0.4 in 3 patients (10.7%). Visual acuity in the affected eye was accurate light projection in 3 patients (10.7%), and inaccurate light projection in one patient (3.6%).
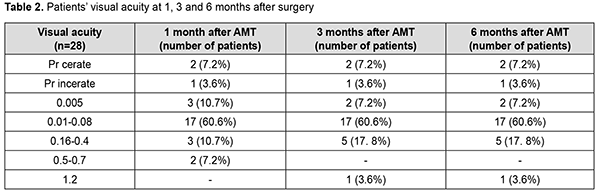
Two-layer amniotic membrane graft was used during surgery in 27 patients (96.4%), and three-layer amniotic membrane graft, in one patient (3.6%). At discharge, the conjunctiva was pink in 24 patients (85.7%), and moderately hyperemic in 4 patients (14.3%). In addition, corneal stromal edema was moderate in 25 patients (89.2%), apparent in 2 patients (7.2%) and no edema was seen in one patient (3.6%). The amniotic graft was unaffected in 26 patients (92.8%) and appeared partially lysed in 2 patients (7.2%). Moreover, it was semitransparent in all the 28 patients (100%). At discharge, visual acuity was 0.005 in 2 patients (7.2%), 0.01 to 0.08 in 16 patients (57.1%), 0.1 to 0.14 in 2 patients (7.2%), and 0.2 to 0.4 in 4 patients (14.2%). Visual acuity in the affected eye was accurate light projection in 3 patients (10.7%), and inaccurate light projection in one patient (3.6%). At 1 month after surgery, the amniotic membrane was unaffected in 26 patients (92.8%) and appeared partially lysed in 2 patients (7.2%). In addition, visual acuity was 0.005 in 3 patients (10.7%), 0.01 to 0.08 in 17 patients (60.6%), 0.16 to 0.4 in 3 patients (10.7%), and 0.5 to 0.7 in 2 patients (7.2%). Visual acuity in the affected eye was accurate light projection in 2 patients (7.2%), and inaccurate light projection in one patient (3.6%). Table 2 shows changes in visual acuity over time in study patients. At 1 month after surgery, corneal surface was epithelialized in 25 patients (89.3%), and corneal epitheliopathy was seen in 3 patients (10.7%). Vascularization of the limbus was seen in 15 patients (53.5%), vascularization of two corneal quadrants, in 11 patients (39.3%), total corneal vascularization, in 1 patient (3.6%), and no corneal vascularization, in 3 patients (10.7%). Punctuate infiltration of the cornea was noted in 1 patient (3.6%) and no corneal infiltration, in 27 patients (96.4%). Corneal stromal edema was seen in 24 patients (85.7%) and no corneal stromal edema, in 4 patients (14.3%).
At 3 months after surgery, the amniotic membrane was partially lysed in 19 patients (67.8%), totally lysed in 8 patients (28.6%) and unaffected in 1 patient (3.6%). In addition, visual acuity was 0.005 in 2 patients (7.2%), 0.01 to 0.08 in 17 patients (60.6%), 0.16 to 0.4 in 5 patients (17.8%), and 1.2 in 1 patient (3.6%). Visual acuity in the affected eye was accurate light projection in 2 patients (7.2%), and inaccurate light projection in one patient (3.6%). Moreover, corneal surface was epithelialized in 24 patients (85.7%), and corneal epitheliopathy was seen in 4 patients (14.3%). Vascularization of the limbus was seen in 16 patients (57.1%), vascularization of two corneal quadrants, in 9 patients (32.1%), total corneal vascularization, in 2 patients (7.2%), and no corneal vascularization, in one patient (3.6%). No corneal stromal infiltration was seen in all the 28 patients (100%). Corneal stromal edema was moderate in 26 patients (92.8%), and no edema was seen in 2 patients (7.2%). At 6 months after surgery, the amniotic membrane was partially lysed in 4 patients (14.3%) and totally lysed in 24 patients (85.7%). Diffuse corneal opacity was observed in 2 patients (7.2%) and mild focal corneal opacity, in 26 patients (92.8%). In addition, visual acuity was 0.005 in 2 patients (7.2%), 0.01 to 0.08 in 17 patients (60.6%), 0.16 to 0.4 in 5 patients (17.8%), and 1.2 in 1 patient (3.6%). Visual acuity in the affected eye was accurate light projection in 2 patients (7.2%), and inaccurate light projection in one patient (3.6%). Moreover, corneal surface was epithelialized in 27 patients (96.4%), and corneal epitheliopathy was seen in 1 patient (3.6%). Vascularization of the limbus was seen in 15 patients (53.5%), vascularization of two corneal quadrants, in 10 patients (35.7%), total corneal vascularization, in 2 patients (7.2%), and no corneal vascularization, in one patient (3.6%). Neither corneal infiltration nor corneal stromal edema was observed in all the 28 study patients (100%).
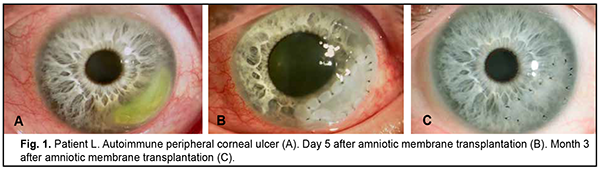
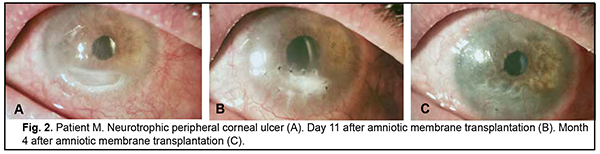
Discussion There is no universal technique for placing the amnion on the wound surface for AMT. There have been numerous contradictory reports concerning the right way to place the AM on the ocular surface. The amnion can be sutured to the ocular surface with its epithelial basement membrane side up and the stromal side down (the preferred technique) or stromal side up [10, 25, 26]. This is important, because the orientation of the AM on the ocular surface should depend on the indication for AMT as well as the desired therapeutic effect. Histopathology has demonstrated that, after AMT, re-epithelization of the corneal surface with host-derived epithelium occurred mostly on the epithelial basement membrane [27]. Nubile and colleagues [28] evaluated integration of AM into the corneal stroma using confocal microscopy and demonstrated that multiple layers of AM can integrate into the corneal stroma. Another confocal microscopy study by Nubile and colleagues [29] found that when the AM acts as a patch, that is epithelial cells migrate under rather than over the membrane, the membrane disintegrates and is lost. With the defect filled with multiple layers of the AM, the membrane is then fixed layer by layer with numerous interrupted sutures. We have previously demonstrated that placing an excessive number of interrupted sutures on the cornea was accompanied by marked inflammatory reaction with corneal infiltration and vascularization as well as the formation of intensive opacity [31, 32]. The method proposed by us consists in forming a two layer or three layer amniotic graft and anchoring it to the surrounding cornea by a row of interrupted 10-0 sutures. It allows to reduce the number of sutures on the cornea twofold or threefold, and correspondently reduce the incidence of corneal trauma and the duration of surgery. Depending on the desired clinical effect (with the amnion used as a temporary patch or integrated into the corneal stroma), the AM may be placed with the epithelial side facing up or down. In the current study, there was a reduction in corneal stromal edema at discharge after AMT by the proposed technique (χ2 = 29.7; p = 0.0005). At 3 months, moderate stromal edema was seen in 2 patients (7.2%), and at 6 months, in no patients. Corneal stromal infiltration resorbed at 1 month after surgery compared to at discharge (χ2 = 9.16; p = 0.0025), and was present in no patients at 3 months. Corneal surface was epithelialized at 1 month after AMT (χ2 = 45.16; p = 0.0005). By 6 months, complete epithelialization of the corneal surface was achieved in 27 patients (96.4%). AMT by the proposed technique facilitated the formation of mild focal corneal opacity in 26 patients (92.8%). Conclusion Our improved AMT technique reduces the number of sutures on the cornea, enables filling the corneal stromal defect and contributes to decreased inflammatory response and early epithelialization of the corneal surface.
Conflict of Interest Statement. The authors declare no conflict of interest. Funding Support. There are no external sources of funding.
References 1.Abdulhalim BE, Wagih MM, Gad AA, Boghdadi G, Nagy RR. Amniotic membrane graft to conjunctival flap in treatment of non-viral resistant infectious keratitis: a randomised clinical study. Br J Ophthalmol. 2015 Jan;99(1):59-63. 2.Grau AE, Duraxn JA. Treatment of a large corneal perforation with a multilayer of amniotic membrane and tachoSil. Cornea. 2012 Jan;31(1):98-100. 3.Liu J, Li L, Li X. Effectiveness of Cryopreserved Amniotic Membrane Transplantation in Corneal Ulceration: A Meta-Analysis. Cornea. 2019;38:454–462. 4.Smal RM. [Pathogenetic grounds for and efficacy of amniotic membrane transplantation for non-infectious corneal ulcers]. [Cand Sc (Med) Thesis]. Odesa: Filatov Institute of Eye Diseases and Tissue Therapy; 2007. Russian. 5.Schroeder A, Theiss C, Steuhl KP, Meller K, Meller D. Effects of the human amniotic membrane on axonal outgrowth of dorsal root ganglia neurons in culture. Curr Eye Res. 2007 Sep;32(9):731-8. 6.Ueta M, Kweon MN, Sano Y. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002 Sep;129(3):464-70. 7.Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of the eye: (Burns of the second degree). Br J Ophthalmol. 1946 Jun;30(6):337-45. 8.Trufanov SV. [Use of human preserved amniotic membrane in ocular reconstructive surgery]. [Abstract of Cand Sc (Med) Thesis]. Moscow: Helmholtz Research Institute of Eye Diseases. Russian. 9.Lacorzana J. Amniotic membrane, clinical applications and tissue engineering. Review of its ophthalmic use. Arch Soc Esp Oftalmol (Engl Ed). 2020 Jan;95(1):15-23. 10.Sabater-Cruz N, Figueras-Roca M, González A, Padró-Pitarch L. Current clinical application of sclera and amniotic membrane for ocular tissue bio-replacement. Cell Tissue Bank. 2020. 2020 Dec;21(4):597-603. 11.Zemanová M, Pacasová R, Šustáčková J, Vlková E. Amniotic membrane transplantation at the department of ophthalmology of the University hospital BRNO. Cesk Slov Oftalmol. Spring 2021;77(2):62-71. 12.Arvola R, Holopainen J. Amnion in the treatment of ocular diseases. Duodecim. 2015;131(11):1044-9. Finnish. 13.Morikawa K, Sotozono C, Inatomi T, et al. Indication and Efficacy of Amniotic Membrane Transplantation Performed under Advanced Medical Healthcare. Nippon Ganka Gakkai Zasshi. 2016 Apr;120(4):291-5. 14.Paolin А, Cogliati E, Trojan D. Amniotic membranes in ophthalmology: long term data on transplantation outcomes. Cell Tissue Bank. 2016 Mar;17(1):51-8. 15.Röck T, Bartz-Schmidt KU, Landenberger J, Bramkamp M. Amniotic Membrane Transplantation in Reconstructive and Regenerative Ophthalmology. Ann Transplant. 2018 Mar 6;23:160-165. 16.Arya SK, Bhala S, Malik A, Sood S. Role of amniotic membrane transplantation in ocular surface disorders. Nepal J Ophthalmol. Jul-Dec 2010;2(2):145-53. 17.Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014 Jun 24;4(2):111-21. 18.Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SC. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008 Aug;126(8):1059-66. 19.Thomasen H, Pauklin M, Steuhl KP, Meller D. Comparison of cryopreserved and air-dried human amniotic membrane for ophthalmologic applications. Graefes Arch Clin Exp Ophthalmol. 2009 Dec;247(12):1691-700. 20.Uhlig CE, Müller VC. Resorbable and running suture for stable fixation of amniotic membrane multilayers: A useful modification in deep or perforating sterile corneal ulcers. Am J Ophthalmol Case Rep. 2018 Apr 19;10:296-299. 21.Kogan S, Sood A, Granick MS. Amniotic Membrane Adjuncts and Clinical Applications in Wound Healing: A Review of the Literature. Wounds. 2018 Jun;30(6):168-173. 22.Dietrich T, Sauer R, Hofmann-Rummelt C, Langenbucher A, Seitz B. Simultaneous amniotic membrane transplantation in emergency penetrating keratoplasty: a therapeutic option for severe corneal ulcerations and melting disorders. Br J Ophthalmol. 2011 Jul;95(7):1034-5. 23.Brücher VC, Eter N, Uhlig CE. Results of Resorbable and Running Sutured Amniotic Multilayers in Sterile Deep Corneal Ulcers and Perforations. Cornea. 2020 Aug;39(8):952-956. 24.Jirsova K, GL Jones. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017 Jun;18(2):193-204. 25.Kasparov AA, Trufanov SV. [Use of preserved amniotic membrane for reconstruction of the surface of the anterior eye segment]. Vestn Oftalmol. May-Jun 2001;117(3):45-7. Russian. 26.Novytskyy IYa. [Place of amniotic membrane transplantation in treatment of corneal diseases accompanied by neovascularization]. Vestn Oftalmol. Nov-Dec 2003;(6):9-11. Russian. 27.Resch MD, Schlötzer-Schrehardt U, Hofmann-Rummelt C, Sauer R, Cursiefen C, et al. Adhesion Structures of Amniotic Membranes Integrated into Human Corneas. Invest Ophthalmol Vis Sci. 2006 May;47(5):1853-61. 28.Nubile M, Dua HS, Lanzini M, et al. In vivo analysis of stromal integration of multilayer amniotic membrane transplantation in corneal ulcers. Am J Ophthalmol. 011 May;151(5):809-822.e1. 29.Nubile M, Dua HS, Lanzini TE, et al. Amniotic membrane transplantation for the management of corneal epithelial defects: an in vivo confocal microscopic study. Br J Ophthalmol. 2008 Jan;92(1):54-60. 30.Sereda EV, Vit VV, Drozhzhina GI, Gaidamaka TB. [Corneal inflammation and proliferative activity of anterior epithelial cells in experimental bacterial keratitis and different types of amniotic membrane fixation]. Oftalmol Zh. 2016;1:36-42. Russian. 31.Sereda EV, Drozhzhina GI, Gaidamaka TB, et al. [Efficacy of different surgical techniques of amniotic membrane transplantation]. Oftalmol Zh. 2016;4:3-10. Russian.
|