J.ophthalmol.(Ukraine).2022;1:37-43.
|
http://doi.org/10.31288/oftalmolzh202213743 Received: 11 August 2021; Published on-line: 15 March 2022 Computed tomography evidence of the state of the paranasal sinuses in patients with uncomplicated anterior uveitis versus anterior uveitis complicated by optic neuritis L. V. Venger 1, O. V. Kovtun 1, V. V. Savko 2 1 Odesa National Medical University; Odesa (Ukraine) 2 SI "The Filatov Institute of Eye Diseases and Tissue Therapyof the NAMS of Ukraine"; Odesa (Ukraine) E-mail: kvnkonovalova@gmail.com TO CITE THIS ARTICLE: Venger L.V., Kovtun O.V., Savko V.V. Computed tomography evidence of the state of the paranasal sinuses in patients with uncomplicated anterior uveitis versus anterior uveitis complicated by optic neuritis. J.ophthalmol.(Ukraine).2022;1:37-43. http://doi.org/10.31288/oftalmolzh202213743 Background: It is clinically important to review the state of patient’s paranasal sinuses, because a paranasal sinus disease creates the conditions for the onset of infection and its spread to the eyes and nose. This is especially important given a significant rise in the incidence of ocular inflammatory disease in recent years. Purpose: To identify computed tomography (CT) features of the paranasal sinuses in patients with idiopathic anterior uveitis. Material and Methods: One hundred and fifty patients with unilateral idiopathic anterior uveitis were involved in the study. Of these, 114 did not have signs of optic neuritis, and 36 had optic neuritis in the presence of uveitis. Patients underwent a routine eye examination (best-corrected visual acuity, ophthalmoscopy, biomicroscopy, intraocular pressure measurement, and Humphrey perimetry). Sinus CT scans were performed on a Philips Brilliance 16-slice scanner. Patients received treatment as per the Protocol for the Diagnosis and Treatment of Anterior Uveitis. Results: On the basis of CT reports, we identified CT features of the paranasal sinuses in patients with idiopathic anterior uveitis complicated by optic neuritis. There was CT evidence of maxillary sinus mucosal thickening and cerebrospinal fluid space expansion in all patients (n=36) with anterior uveitis complicated by optic neuritis. Fluid accumulation in the maxillary, frontal, and sphenoid sinuses was diagnosed in 41.7%, 11.1%, and 5.6%, respectively, of patients with anterior uveitis complicated by optic neuritis, which was significantly higher, than in patients with uncomplicated anterior uveitis. We found a significant and strong positive correlation of (a) maxillary sinus mucosal thickening with fluid accumulation in the maxillary sinus and cerebrospinal fluid space expansion and (b) decreased frontal sinus pneumatization and fluid accumulation in the frontal sinus. Conclusion: Sinus CT data enabled to find a significant association between the pattern of changes in the paranasal sinuses and the development of optic uveitis in patients with idiopathic anterior uveitis, which, generally, argues for performing sanitation of the sinuses when treating ocular inflammation. Keywords: idiopathic anterior uveitis, optic neuritis, paranasal sinuses, computed tomography
Introduction Endogenous uveitis is one of the most severe multifactorial diseases of the eye, characterized by a chronic course with recurrences and complications, with the latter making diagnosis and treatment a challenge. Optic nerve inflammation is found in 14 to 27% of cases and is one of the most severe complications of endogenous uveitis [1-3]. Uveitis may be caused by an inflammatory or infectious disease of the head (particularly, of the paranasal sinuses, teeth and gums, like caries, tooth root granuloma, or periodontosis) and/ or kidneys [4]. Although it is not always possible to determine the etiology of ocular inflammation, it is possible to determine the risk factors for anterior uveitis. We have previously reported that concomitant otorhinological and odontogenic inflammatory diseases were found in 82.5% of patients with anterior uveitis [5], which called for further study of the paranasal sinuses in these patients. Clinical manifestations of uveitis depend on the site of primary inflammation, morphological characteristics, activity and course of the disease [6]. X-ray radiography is a traditional technique that allows assessing the extent of inflammation, particularly, in the various paranasal sinuses. In our previous study of 54 patients with anterior idiopathic uveitis, an examination included, particularly, paranasal sinus radiography. Based on the analysis of clinical characteristics and X-ray images of the sphenoid bone, we found optic neuritis only in 6 patients (15.3%) of the group of individuals with a well-developed sphenoid sinus (i.e., the sinus located from the vertical perpendicular from the tangent line to the midpoint of the pituitary fossa floor to the cross-point with the projection of the sella turcica diaphragm), but not in patients of the spongious and pseudo-spongious group [7]. Previous studies by others also demonstrated a relationship between paranasal sinus inflammation and ocular inflammatory lesion. Thus, Panphilova and Shpak [8] noted a frequent association between hyperpneumatization of the sphenoid bone and inflammation involving either both the optic nerve and retina or optic nerve only, in the form of optic nerve neuritis or atrophy [9]. Acute inflammation in the proximity of the nasal cavities is a serious issue in pediatric otorhinolaryngology, contributes to the development of difficult-to-treat forms of sinusitis and orbital complications which may lead to total visual loss and the development of severe intracranial complications in the child [10]. We have failed to find any reports on (a) the role of paranasal sinus inflammation in the course of uveitis, (b) the odds of the development of complications, or (c) the value of X-ray radiography, computed tomography (CT) and/or magnetic resonance imaging (MRI) in detecting the pathologic changes in the paranasal cavity in uveitis. A study on ultrasound-based assessment of optic disc diameter in patients with ocular contusion [11] demonstrated the value of this technique, whereas a study on the use of multislice CT in the diagnosis of mixed traumatic brain injury showed the technique to be promising for measurements of cross-section diameter of the optic nerve in these patients [12]. The X-ray radiography allows identifying patients with increased risk of optic nerve inflammation among those with idiopathic anterior iridocyclitis, but the identification is not always significant. Given this fact, we believe it is reasonable to use CT for studies on pathologic changes in the paranasal sinuses, because it is a more advanced and informative method providing higher definition imaging with no shadows from organs. The purpose of this study was to identify CT features of the paranasal sinuses in patients with idiopathic anterior uveitis, either uncomplicated or complicated by optic neuritis. Material and Methods This open-label and non-interventional study was conducted within the framework of the research program of the Filatov Institute of Eye Disease and Tissue Therapy. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. One hundred and fifty patients (94 men and 56 women; age, 18 to 83 years) with unilateral idiopathic anterior uveitis who were examined and treated in the Department of Ocular Inflammation, the Filatov Institute of Eye Disease and Tissue Therapy, and Medical Eye Care Center of Odesa National Medical University, were involved in the study. The inclusion criterion was the presence of unilateral idiopathic anterior uveitis. The exclusion criteria were diabetes; acute infectious, viral, or cardiovascular disease; abnormal circulation in the major ocular vessels; history of ocular surgery; or pregnancy. Anterior uveitis was diagnosed using International Classification of Diseases, 10th Revision (ICD-10) criteria and based on the Standardization of Uveitis Nomenclature (SUN) criteria, categorizing uveitis along several dimensions: course, laterality, anatomic location of the inflammation, morphology, and presence of active infection [13]. Patients underwent a routine eye examination (best-corrected visual acuity, ophthalmoscopy, biomicroscopy, intraocular pressure (IOP) measurement, and Humphrey perimetry). Sinus CT scans were performed on a Philips Brilliance 16-slice scanner (Philips Medical Systems, Best, the Netherlands). A protocol with 0.5-1 mm slice thickness, field of view of 10 cm, tube voltage of 120 kVp, and tube current of 300 mAs per slice, was utilized. Both soft tissue and bone reconstruction filters were applied. The patient's head was accommodated and fixed on a head rest. To perform the first slice series, the head was scanned in helical mode without gantry tilt (Phase 1, non-contrast). Axial images and, if required, coronary images, were obtained. The mucous membrane thickening of ≤ 3 mm, in the absence of pathological nasal symptoms, was not clinically significant, since the thickness of the nasal and sinus mucous membrane oscillates with time, with a period of one to six hours. We have compared the 150 patients with unilateral idiopathic anterior uveitis with those 36 patients (of the 150) who had optic neuritis in the presence of uveitis with regard to CT data. The features of the clinical picture in both study groups were reported previously [5]. Patients received treatment in cooperation with an otorhinolaryngologist as per the Protocol for the Diagnosis and Treatment of Anterior Uveitis which was approved on the basis of United States Public Health Service (USPHS)/ Infectious Diseases Society of America (IDSA) Guidelines for the Prevention of Opportunistic Infections in Persons Infected with Human Immunodeficiency Virus. Treatment involved broad-spectrum antibiotics, non-steroidal anti-inflammatory drugs (administered electrophoretically, by topical instillation, and/or internally), corticosteroids (intramuscularly and/or parabulbarly), biological immune response modulators, papillary massage, antibacterial substances delivered via nasal electrophoresis, antioxidants, dehydratation therapy, and desensitizing therapy [14, 15]. The average treatment duration ranged from 10 to 12 days. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Pearson chi-square test and Spearman rank correlation coefficient were used to assess associations between study parameters [16]. Results We have previously demonstrated [5] that concomitant otorhinolaryngological and/or odontogenic inflammatory diseases were found in 82.5% of patients with anterior uveitis. In addition, there was a significant association between the presence of concomitant otorhinolaryngological and odontogenic inflammatory diseases and the development of optic neuritis in patients with anterior uveitis (χ2=5.50, p=0.0191) [5]. This called for further study of the maxillary, frontal and sphenoid sinuses in patients with anterior uveitis, either uncomplicated or complicated by optic neuritis, based on CT protocols. There was CT evidence of maxillary sinus mucosal thickening in 39 patients, including all patients (n=36) with anterior uveitis complicated by optic neuritis and only 3 of the 114 patients (2.6%) with uncomplicated anterior uveitis. In addition, there was CT evidence of fluid accumulation in the maxillary sinuses in 15 of the 36 (41.7%) patients with anterior uveitis complicated by optic neuritis, and only 5 of the 114 patients (4.4%) with uncomplicated anterior uveitis. The percentage frequency of CT evidence of fluid accumulation in the frontal sinus was significantly higher for patients with anterior uveitis complicated by optic neuritis (11.1%) than for patients with uncomplicated anterior uveitis (0.9%; or 1 of 114). Moreover, there was CT evidence of decreased frontal sinus pneumatization in 3 of the 114 patients (2.6%) with uncomplicated anterior uveitis compared to 11 of the 36 patients (30.6%) with anterior uveitis complicated by optic neuritis. This may be used as an additional criterion for identifying the presence of pathological inflammation and predicting the probability or beginning early treatment of the optic nerve. The percentage frequency of CT evidence of fluid accumulation in the sphenoid sinus was similar to that in the frontal sinus. In addition, it was higher for patients with anterior uveitis complicated by optic neuritis (5.6%; or 2 of 36) than for patients with uncomplicated anterior uveitis (0.9%; or 1 of 114). There was CT evidence of cerebrospinal fluid space expansion in 3 of the 114 patients (2.6%) with uncomplicated anterior uveitis compared to 36 of the 36 patients (100%) with anterior uveitis complicated by optic neuritis. There was a significant correlation among several CT measurements (Table 1). Thus, a significant strong correlation between maxillary sinus mucosal thickening and fluid accumulation in the maxillary sinus (r = 0.549, p < 0.0001) as well as cerebrospinal fluid space expansion (r = 0.896, p < 0.0001) was identified. We also found a moderate positive correlation between cerebrospinal fluid space expansion and fluid accumulation in the maxillary sinus (r = 0.418, p < 0.0001). In addition, there was a statistically significant but mild positive correlation between fluid accumulation in the frontal sinus and maxillary sinus mucosal thickening (r = 0.228, p < 0.005) as well as cerebrospinal fluid space expansion (r = 0.229, p < 0.005). Moreover, we found a significant and strong positive correlation between decreased frontal sinus pneumatization and fluid accumulation in the frontal sinus (r = 0.579, p < 0.000) and a significant and moderate positive correlation between decreased frontal sinus pneumatization and maxillary sinus mucosal thickening (r = 0.436, p < 0.000) as well as fluid accumulation in the maxillary sinus (r = 0.465, p < 0.000).
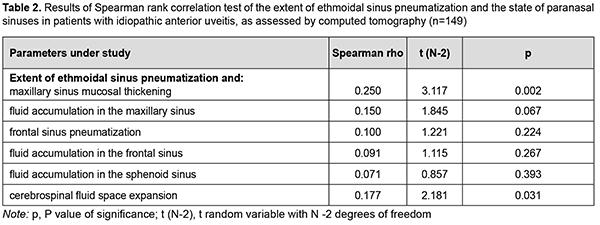
A statistically significant but mild positive correlation between CT evidence of the extent of ethmoidal sinus pneumatization and maxillary sinus mucosal thickening (r = 0.250, p = 0.002) as well as cerebrospinal fluid space expansion (r = 0.177, p = 0.031) was identified. However, no correlation was found between CT evidence of the extent of ethmoidal sinus pneumatization and any other CT measures like fluid accumulation in the maxillary sinus, frontal sinus pneumatization, fluid accumulation in the frontal sinus, or fluid accumulation in the sphenoid sinus (Table 2).
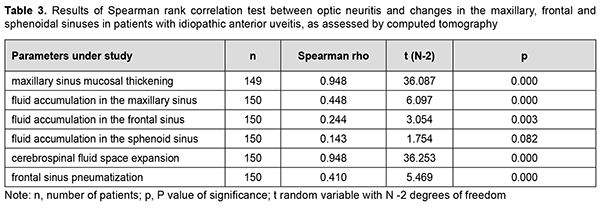
We also examined the presence of a relationship between optic neuritis and CT evidence of the state of the maxillary and frontal sinuses in patients with anterior uveitis, and found (a) a significant and strong positive correlation with maxillary sinus mucosal thickening and cerebrospinal fluid space expansion (n=150; r = 0.948, p = 0.000), (b) a significant and moderate positive correlation with fluid accumulation in the maxillary sinus (n=150; r = 0.448, p = 0.000) and decreased frontal sinus pneumatization (n=150; r = 0.410, p = 0.000), and (c) a significant but mild positive correlation with fluid accumulation in the frontal sinus (n=150; r = 0.244, p = 0.003). However, no correlation was found between optic neuritis and CT evidence of fluid accumulation in the sphenoid sinus in patients with anterior uveitis (n=150; r = 0.143, p = 0.082) (Table 3).
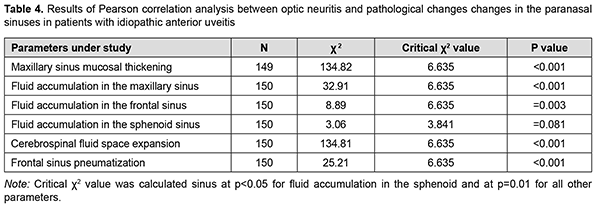
The results of Pearson correlation analysis are presented in table 4.
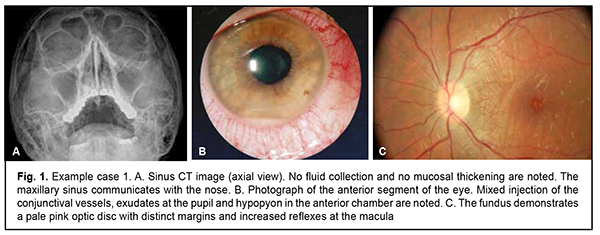
There was evidence of significant correlation between the risk factor (i.e., the presence of pathological changes in the paranasal sinuses) and the outcome (i.e., optic neuritis) in patients with anterior uveitis. Therefore, a significant correlation established between the presence of pathological changes in the paranasal sinuses and optic neuritis in patients with anterior uveitis supposed that paranasal sinus disease is a serious risk factor for complications in anterior uveitis. The below examples illustrate the results obtained in the study. Example 1. A 47-year-old male patient with anterior uveitis OD had uncorrected visual acuity of 0.4 OD and 1.0 OS, for the first time presented with the disease, and had no concomitant disease (Fig. 1). After a course of anti-inflammatory therapy, uncorrected visual acuity was 0.1 OU, and there was complete relief of the clinical signs of inflammation.
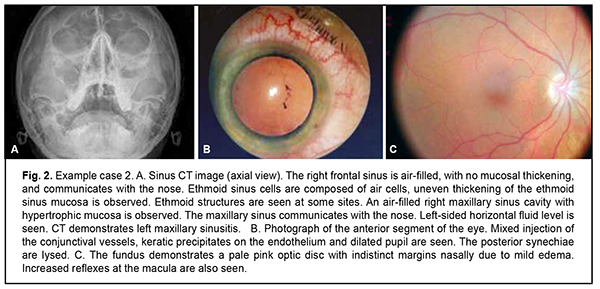
Example 2. A 43-year-old female patient with anterior uveitis OS had uncorrected visual acuity of 1.0 OD and 0.3 OS, for the first time presented with the disease, and had no concomitant disease (Fig. 2). After a course of anti-inflammatory therapy, uncorrected visual acuity was 0.1 OU, there was complete relief of the clinical signs of inflammation and the posterior synechiae were lysed.
Discussion We identified a direct association between inflammation of both the uvea and optic nerve and paranasal sinus disease. This is in agreement with previous reports that acute nasal cavity inflammation is a serious problem contributing to the development difficult-to-treat forms of sinusitis and orbital complications which may lead to total visual loss and the development of optic nerve complications [7, 10]. Panfilova and colleagues conducted research at the Filatov Institute of Eye Disease and Tissue Therapy [17], and their research and review on the literature drew attention to the fact that sphenoid sinus disease may cause optic neuritis and atrophy. X-ray studies have found changes in the nasal accessory sinuses in some of the diseases leading to uveitis (like Wegener's Granulomatosis and odontogenic uveitis). Anatomical features of the structure of paranasal sinuses and sphenoid bone play an important role in the spread of inflammation in the eye [8]. In some cases, an additional air cavity forms between the anterior fossa and the orbit, which, in the presence of inflammation and a well-developed ocular cavity with the optic nerve canal and superior orbital fissure, may facilitate the spread of inflammation to the orbit and cranial cavity. It has been reported that, in some patients with anterior uveitis, a sphenoid pouch forms in the postero-superior angle of the maxillary sinus and located upwards and runs along the lamina papyracea and sphenoidal process of the palatine bone to the anterior surface of the sphenoid bone. In most cases, the pouch makes contact with the posterior ethmoid bone, which facilitates the spread of inflammation from one sinus to another. In the presence of sphenoethmoid cells (Onodi cells) spreading posteriorly, superiorly and laterally to the ethmoid sinus (i.e., the optic nerve may course through the Onodi cell), there is a risk of inflammation of the optic nerve and internal carotid artery. In the presence of a well-developed sphenoid sinus, its walls become thinned, and the optic nerve is in close proximity to the sinus, thus facilitating direct spread of inflammation and infection from the sinus to the optic nerve; this explains the mechanism of optic neuritis in this category of patients [18-21]. Therefore, the presence of the above anatomic features facilitates rapid spread of inflammation with a potential to cause acute anterior uveitis, either alone or complicated by optic neuritis. Generally, the results obtained argue for performing sanitation of the paranasal sinuses when treating ocular inflammation. References 1.Panchenko MV, Samofalova MN, Honchar ON, Lytvyshchenko AV, Friantseva MV. [Retinal nerve fiber layer thinning in uveitis complicated by optic nerve inflammation]. Arkhiv oftalmologii Ukrainy. 2016;4(1):50-3. Russian. 2.Gupta A, Gupta V, Herbort CP, Khairallah M, editors. Uveitis Text and Imaging. New Delhi: Jaypee Brothers Medical Publishers; 2009. 3.Nussenblatt RB, Whitcup SM, editors. Uveitis: fundamental and clinical practice. 4th ed. Mosby; St. Louis: 2012. 4.Tubulointerstitial nephritis and uveitis (TINU) syndrome: a systematic review of its epidemiology, demographics and risk factors. Orphanet J Rare Dis. 2017; 12(1):128. 5.Venger LV, Savko VV, Kovtun OV, Sokolov VM. Clinical features of the course of optic neuritis as a complication of idiopathic anterior uveitis. J.Ophthalmol.(Ukraine). 2021;(5):41-7. 6.Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. 2nd ed. New Delhi: Jaypee Brothers; 2013. 7.Venger LV, Kovtun OV, Savko VV. [Relationship between the size of the ethmoid sinus and the possibility of developing optic neuritis in patients with idiopathic anterior iridocyclitis]. In: [Proceedings of the national Conference on Current Issues in Ophthalmology]. Mykolaiv, September 22-23, 2021. p. 33-5. Ukrainian. 8.Panfilova GV, Shpak NN. [Value of the sphenoid sinus size in the development of optic nerve disease]. Oftalmol Zh. 1993:185-8. Russian. 9.Zolotareva MM. [Ocular symptoms in diseases of the eye, nose, throat and oral cavity]. Minsk: Belarus; 1969. Russian. 10.Garashchenko TE, Kitaigorodskii AP. [Diagnosis and treatment of orbital complications of acute and chronic sinusitis in children]. Rossiiskaia rinologiia. 1996;(2-3):74-6. Russian. 11.Zelentsov KS, Ioileva EE, Zelentsov SN. [Ultrasound assesment of optic disc size in patients with ocular contusion]. Tochka Zreniya. Vostok – Zapad. 2017; (3):78-80. Russian. 12.Semenov AV, Monakov NV, Balkhanova EI, Raznobarskii AA, Mamonova TA. [Multislice computed tomography in the diagnosis of mixed traumatic brain injury]. Vestnik rentgenologii i radiologii. 2018;99(3):119-124. Russian. 13.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005 Sep; 140(3):509 -16. 14.Beck RW, Cleary PA, Anderson MM et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326(9): 581–88. 15.Nakagawa H, Noma H, Kotake O, Motohashi R, Yasuda K, Shimura M. Optic neuritis and acute anterior uveitis associated with influenza A infection: a case report. Int Med Case Rep J. 2017;(10):1–5. 16.Glantz S. [Primer of biostatistics]. Moscow: Praktika; 1998. Russian. 17.Panfilova GV, Shitova IIa. [X-ray studies in ophthalmology]. Kyiv: Zdorov’ia. 1980. Russian. 18.Al-Abri R, Bhargava D, Al-Bassam W, Al-Badaai Y, Sawhney S. Clinically significant anatomical variants of the paranasal sinuses. Oman Med J. 2014; 29: 110–13. 19.Albalwi Y, Alroqi A, Alharethy S. Anatomical variations in Computerized Tomography of paranasal sinuses in a Saudi population. Saudi J Otolaryngol Head Neck Surg. 2019;21:1–5. 20.Berhouma M, Jacquesson T, Abouaf L, Vighetto A, Jouanneau E. Endoscopic endonasal optic nerve and orbital apex decompression for nontraumatic optic neuropathy: surgical nuances and review of the literature. Neurosurg Focus. 2014; 37(4):E19. 21.Girguis-Bucher A, Schlegel-Wagner C. Anatomical variation of the extracranial course of the optic nerve in the floor of the sphenoid sinus: first reported case. J Laryngol Otol. 2013 Aug;127(8):822-4.
Conflict of Interest Statement: The authors declare no conflict of interest. Source of Funding: There are no external sources of funding.
|