J.ophthalmol.(Ukraine).2022;1:30-36.
|
http://doi.org/10.31288/oftalmolzh20223036 Received: 11 January 2022; Published on-line: 15 March 2022 Efficacy of the modified staged method of surgical treatment for proliferative diabetic retinopathy Vira S Ponomarchuk, A. R. Korol, M. M. Umanets SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: v.zavodnaya@gmail.com TO CITE THIS ARTICLE: Ponomarchuk Vira S, Korol A. R., Umanets M. M. Efficacy of the modified staged method of surgical treatment for proliferative diabetic retinopathy. J.ophthalmol.(Ukraine).2022;1:30-6. http://doi.org/10.31288/oftalmolzh20223036 Background. Anti-vascular endothelial growth factor (VEGF) agents have been successfully used to reduce the incidence of perioperative and postoperative complications in patients with proliferative diabetic retinopathy (PDR). Purpose: To evaluate the efficacy of a modified staged method of surgical treatment for PDR which involves intravitreal aflibercept (IVA) injection 1-mg prior to vitrectomy. Material and Methods: This study involved 75 patients (75 eyes) with PDR. Group 1 (the control group) received vitrectomy only; Group 2, IVA 1.0 mg prior to vitrectomy; and Group 3, IVA 2.0 mg prior to vitrectomy. Results: Mean visual acuity at 2 months and 6 months was significantly better in eyes treated with aflibercept than in those not treated with aflibercept (p1-2 = 0.0001; p1-3 = 0.0001). In the early postoperative period, transient vitreous hemorrhage was observed significantly more frequently in the control group than in group 2 (p1-2 = 0.0003) or group 3 (p1-3 = 0.0004). The incidence of transient vitreous hemorrhage within 2 months after vitrectomy was also significantly higher in the control group than in group 2 (p1-2=0.09) or group 3 (p1-2=0.017). By 6 months after surgery, rubeosis developed in 5 eyes (16.1%) of the control group, and in no eye of the 44 eyes that received intravitreal aflibercept (р= 0.02). Conclusion: Our modified staged method of surgical treatment for PDR enabled an improvement in visual acuity at 2 months and 6 months after surgery; a reduction in the incidence of transient vitreous hemorrhage in the early postoperative period; and reduction in the risk of the development of iris rubeosis in the late period after surgery. Keywords: proliferative diabetic retinopathy, intravitreal aflibercept, vitrectomy
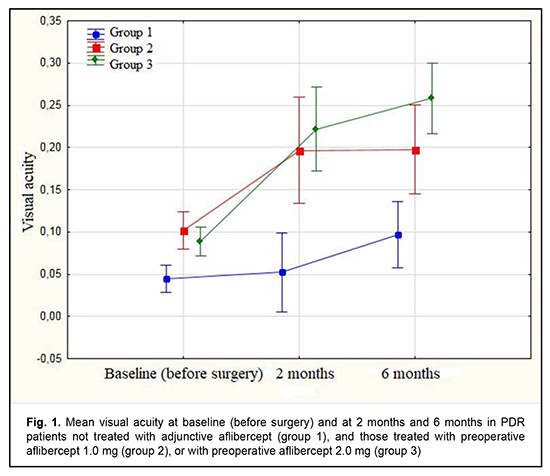
Diabetic retinopathy (DR) is the leading cause of blindness in working-age populations in developed countries [1]. The disease is characterized by progressive retinal ischemia, leading to the development of neovascularization of the retina, optic disc and iris, and formation of epiretinal fibrovascular membranes (ERM). At the later stages of DR, ERM contraction is accompanied by tractional or combined tractional and rhegmatogenous retinal detachment. Although advances in vitreoretinal surgery have improved the anatomical and functional outcomes of vitrectomy for severe complications of proliferative diabetic retinopathy (PDR) [2], PDR surgery is still associated with a high rate of perioperative and postoperative hemorrhagic complications [3]. Intraoperative hemorrhage makes visualization of the operative field and precise surgical manipulations difficult, and may be accompanied by serious complications [4, 5]. Vitreous hemorrhage after surgery for DR has been reported to occur in 30%-75% of eyes and may be divided into early (≤ 4 weeks) and late (> 4 weeks) [2-5]. Early transient vitreous hemorrhage may result from remnants of fibrivascular ERMs, whereas late transient vitreous hemorrhage, from neovascularization at the site of sclerotomy, vitreous base, iris, anterior chamber angle and retina. Peripheral retinal cryotherapy, cryotherapy of the anterior retina and sclerotomy sites, intravitreal infusion of 10% C3F8 or silicone oil, and other methods have been proposed to reduce the risk of postoperative hemorrhagic complications [6-8]. Anti-vascular endothelial growth factor (VEGF) agents like ranibizumab, bevacizumab and aflibercept are commonly used for the treatment of diabetic macular edema and wet age-related macular degeneration (AMD) [9 , 10]. There have been numerous reports on the successful use of anti-VEGF agents for preventing perioperative and postoperative hemorrhagic complications in patients with PDR [11-16]. We have previously demonstrated the potential of our modified staged method of surgical treatment (intravitreal aflibercept injection 1-mg prior to vitrectomy) for PDR, which was found to be as efficacious as the surgical treatment with a conventional (2.0 mg) dose of intravitreal aflibercept, with a reduced incidence of perioperative hemorrhagic complications and a reduced surgical time [17]. In this connection, the purpose of this study was to assess the efficacy of our modified method of staged surgical treatment (involving intravitreal aflibercept injection 1-mg prior to vitrectomy) for PDR. Material and Methods This was an open prospective interventional study of 75 patients (75 eyes) aged 18 to 58 years with PDR. There were 27 men (36%) and 48 women (64%). Of the 75 patients, 31 had type 1 diabetes mellitus (T1DM), and 44, type 2 DM (T2DM). Of the 44 patients with T2DM, 14 required insulin therapy. Diabetes duration was ≤10 years in 11 patients and 10 to 20 years in 64 patients. All the 75 eyes had fibrovascular ERM with a marked proliferative component [18]. Tractional retinal detachment (TRD) threatening the macula was seen in 26, TRD with macular involvement, in 23 eyes, and combined tractional and rhegmatogenous retinal detachment (TRRD), in 6 eyes. No retinal detachment was observed in 20 eyes. Of the 75 eyes, 31 had and 44 had not a history of panretinal laser photocoagulation. On admission, initial complicated cataract was observed in 41 eyes, and 34 eyes had an intraocular lens (IOL) implanted. Patients underwent an eye examination which included visual acuity assessment, refractometry, tonometry, static automated perimetry, biomicroscopy, gonioscopy and ophthalmoscopy. Patients with a history of vitrectomy or anti-VEGF injections, or the presence of uveitis (or intraocular inflammation), iris rubeosis, elevated IOP, total vitreous hemorrhage, central retinal vein or branch occlusion, or central retinal artery occlusion were excluded from the study. Baseline intraocular pressure (IOP) ranged from 18.0 mm Hg to 23.0 mmHg. Patients were divided into three groups. Group 1 (31 eyes) received vitrectomy only; group 2 (17 eyes), intravitreal aflibercept 1.0 mg 3-5 days prior to vitrectomy (our modified method of staged surgical treatment); and group 3 (27 eyes), conventional intravitreal aflibercept 2.0 mg (aflibercept was injected into the vitreous in a routine manner by inserting a 29-gauge needle through the pars plana ciliaris) 3-5 days prior to vitrectomy. Patients of groups 2 and 3 underwent a 25G three-port vitrectomy using an Alcon Constellation 25-G vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA) and OMS-800 OFFISS microscope (Topcon, Tokyo, Japan) on day 3 to day 5 after intravitreal aflibercept injection. Core and peripheral vitrectomy and posterior hyaloid peeling and excision over 3600 were performed using a wide angle viewing system BIOM system (Oculus, Wetzlar, Germany). Epiretinal membranes were removed completely, and endolaser photocoagulation was performed. Surgery was completed with intravitreous tamponade with air only (27 eyes), a mixture of air and perfluoropropane (27 eyes), or silicone oil (21 eyes) [17]. Follow-up duration was 6 months. Before treatment, informed consent was signed by all patients, and they were educated about the possible consequences and complications of the surgical procedure. The study protocol conformed to the tenets of the Declaration of Helsinki, and was approved by the Bioethics Committee of the Filatov Institute (Protocol No. 1 dated October 15, 2018). Written informed consent was obtained from all patients. Visual acuity and the incidence of transient vitreous hemorrhage, rhegmatogenous retinal detachment, and iris rubeosis were assessed in the early postoperative period (< 4 weeks after surgery) and 2 and 6 months after vitrectomy. The non-parametric Chi-squared test was used to analyze contingency tables and to compare proportions. To analyze changes in visual acuity, analysis of variance (ANOVA) for repeated measurement was used, followed by the least significant difference (LSD) test. The level of significance p < 0.05 was assumed. Statistica 8 (StatSoft, Tulsa, OK, USA) software was used for statistical analysis. Results The mean age of patients in group 1 was 59.5 ± 2.28 years versus 40.9 ± 3.90 years for those in group 2 and 45.4 ± 3.38 years for those in group 3. The numbers and proportion of women were greater than those of men in all the groups (23 (74.2%) versus 8 (25.8%) in group 1, 14 (51.9%) versus 13 (48.1%) in group 2, and 11 (64.7%) versus 6 (35.3%) in group 3). In groups 1, 2 and 3, the numbers and proportion of patients with T1DM were 8 (25.8%), 12 (44.4%), and 11 (64.7%), respectively; the numbers and proportion of patients with T2DM, 23 (74.2%), 15 (55.6%), and 6 (35.3%), respectively; and the numbers and proportion of patients with T2DM requiring insulin therapy, 7 (30.4%), 6 (40%) and 1 (16.7%), respectively. The numbers and proportion of patients with diabetes duration of ≤10 years in the study population were 4 (12.9%), 2 (11.8%), and 5 (18.5%), respectively; and the numbers and proportion of patients with diabetes duration of 10 to 20 years, 27 (81.7%), 22 (81.5%), and 15 (88.2%), respectively. Moreover, the numbers and proportion of eyes with a history of panretinal laser photocoagulation in groups 1, 2 and 3 were 7 (22.6%), 15 (55.6%) and 9 (52.9%), respectively. Although the 75 patients had arterial hypertension of grades 1 to 3, it was compensated by the time of surgery. In groups 1, 2 and 3, the baseline visual acuity was 0.04 ± 0.01, 0.1 ± 0.01, and 0.09 ± 0.01, respectively. Figure 1 shows the visual acuity in patients with PDR at baseline and at 2 and 6 months after vitrectomy. There was no significant difference in visual acuity among the three groups at baseline (Fig. 1). The baseline visual acuity was better in groups 2 and 3 (i.e., aflibercept groups) than in group 1, but the differences were not significant (p1-2 = 0.061 and p1-3 = 0.098, respectively).
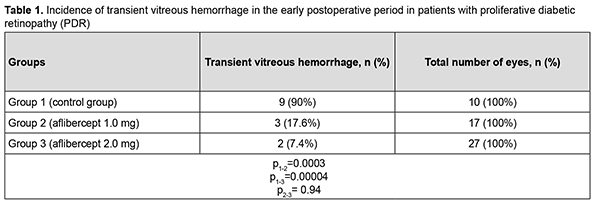
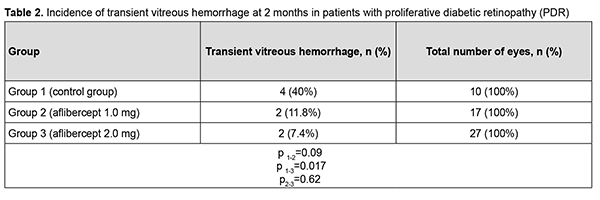
At 2 months after surgery, visual acuity improved from 0.04 at baseline to 0.05 in group 1 (the improvement was not significant, p = 0.72). In addition, visual acuity improved from 0.10 at baseline to 0.20 in group 2, and from 0.09 at baseline to 0.22 in group 3, and these improvements were significant (p = 0.002 and p = 0.0001, respectively). Therefore, a significant improvement in visual acuity was observed as early as 2 months after either vitrectomy with preoperative intravitreal aflibercept 1-mg (our modified method of staged surgical treatment) or vitrectomy with a conventional preoperative intravitreal aflibercept 2-mg. At 6 months after surgery, visual acuity improved from 0.04 at baseline to 0.10 in group 1 (p = 0.02), from 0.10 at baseline to 0.20 in group 2 (p = 0.001), and from 0.09 at baseline to 0.26 in group 3 (p = 0.0001). In addition, mean visual acuity was significantly better in eyes treated with aflibercept than in those not treated with aflibercept at 2 months (p1-2 = 0.0001) and 6 months (p1-3 = 0.0001). There was, however, no significant difference in visual acuity among groups 2 and 3 at 2 months (p2-3 = 0.419) and 6 months (p2-3 = 0.053). In group 1, surgery was completed with intravitreous tamponade with a mixture of perfluoropropane and air (15% C3F8) in 9 eyes (29.0%), 5700-cSt silicone oil tamponade in 21 eyes (67.7%), and air only in 1 eye (3.2%). In addition, surgery was completed with intravitreous tamponade with a mixture of perfluoropropane and air (15% C3F8) and with air only in 8 eyes (47.1%) and 9 eyes (52.9%), respectively, of group 2, and in 10 eyes (37.0%) and 17 eyes (63.0%), respectively, of group 3. Patients that received silicone oil tamponade of the vitreous cavity were excluded from group 1 (the control group) in order to assess the incidence of transient vitreous hemorrhage in the early postoperative period, at 2 months and at 6 months (Tables 1-3). Therefore, in the early postoperative period, transient vitreous hemorrhage was observed significantly more frequently in the control group than in the intravitreal aflibercept 1.0 mg group (p1-2 = 0.0003) or intravitreal aflibercept 2.0 mg group (p1-3 = 0.0004). In the early postoperative period, there was no significant difference in the incidence of transient vitreous hemorrhage between groups 2 and 3 (p2-3 = 0.94). The numbers and proportion of the eyes which developed transient vitreous hemorrhage within 2 months after vitrectomy with tamponade using either a mixture of perfluoropropane and air or air only, was 4 of 10 eyes (40%) for group 1 (the control), compared to only 2 of 17 eyes (11.8%) for group 2, and 2 of 27 eyes (7.4%) for group 3 (p1-2 = 0.09, p1-3 = 0.017), with no significant difference in the incidence of transient vitreous hemorrhage at 2 months between groups 2 and 3 (p2-3=0.62).
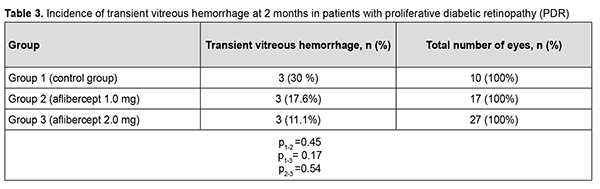
Therefore, at 6 months after vitrectomy for PDR, there was no significant difference in the incidence of transient vitreous hemorrhage between patient groups receiving different doses of aflibercept. The numbers and proportion of the eyes which developed rhegmatogenous retinal detachment within 6 months after vitrectomy were 4 (12.9%) for group 1, 1 (5.9%) for group 2, and 2 (7.4%) for group 3 (р = 0.66). Therefore, at 6 months after vitrectomy for PDR, there was no significant difference in the incidence of rhegmatogenous retinal detachment among the study groups. Of the 75 study eyes, no eye developed iris rubeosis by 2 months after surgery. By 6 months after surgery, rubeosis developed in 5 eyes (16.1%) of the control group, and in no eye of the 44 eyes that received intravitreal aflibercept (р= 0.02). Discussion Vascular endothelial growth factor regulates angiogenesis and vascular permeability and plays a key role in the pathogenesis of diabetic retinopathy and other retinal vascular disorders [9, 19]. Anti-VEGF agents have been successfully used for neovascularization regression and reduction of macular edema, especially in the treatment of intraocular hemorrhage, subretinal neovascular membrane and diabetic macular edema [10, 20]. Chen and Park [11] were the first to report the use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe PDR and hypothesized that a decrease in PDR epiretinal membrane vascularity (neovascular regression) may reduce the risk of hemorrhagic complications [11]. Others [12–16, 21–23] subsequently reported that preoperative intravitreal bevacizumab reduces the incidence of postoperative complications due to obliteration of newly formed vessels in epiretinal membranes. It has been noted that preoperative intravitreal bevacizumab substantially facilitated segmentation and delamination of fibrovascular ERM, and contributed to improved visualization during surgery. Another advantage of intravitreal bevacizumab was a reduced incidence of the use of endodiathermy as well as a reduced surgical time [13]. In addition, there was a reduced incidence of vitreous hemorrhage, especially in the early postoperative period, in patients that received preoperative intravitreal bevacizumab. Velazquez and colleagues [24], however, reported that, in patients with PDR being treated with anti-VEGF agents prior to vitrectomy, increased ERM contraction and retinal traction has frequently been observed, leading to the progression of tractional retinal detachment and even the development of retinal tear [24]. Similar results were reported by Zhao and colleagues [25], who noted that such complications mostly developed 10-14 days after intravitreal bevacizumab injection. We hypothesized that our modified staged surgical treatment of patients with PDR (with a dose of aflibercept reduced to 1 mg) may allow obliteration of newly formed vessels in epiretinal membranes, with a reduction in the incidence of complications (like the progression of tractional retinal detachment and the development of retinal tears) associated with ERM contraction after injection of anti-VEGF. This hypothesis was confirmed in our more recent works. Obliteration of newly formed vessels in epiretinal membranes was observed on day 5 in patients with PDR treated with intravitreal aflibercept injection 1.0 mg as well as in those treated with intravitreal aflibercept injection 2.0 mg. There was, however, ophthalmoscopic evidence of increased ERM contraction with an increased traction component in (a) 23 eyes (85.2%) treated with intravitreal aflibercept injection 2.0 mg, leading to the development of retinal tear in 2 eyes (7.4%) and (b) 3 eyes (17.6%) treated with intravitreal aflibercept injection 1.0 mg, which did not lead to the development of retinal tear in any eye. We have also found that the use of intravitreal aflibercept injection 1.0 mg as a preoperative adjunct before vitrectomy surgery was as efficacious as the use of conventional intravitreal aflibercept injection 2.0 mg with regard to the reduction in the risk of hemorrhagic complications, need for silicone oil tamponade, and, consequently, reduced vitrectomy time [17]. The results of the current study demonstrate that, in eyes treated with our modified staged method (the use of intravitreal aflibercept injection 1.0 mg as a preoperative adjunct before vitrectomy surgery), visual acuity significantly improved at 2 and 6 months after surgery, which was comparable to the use of conventional intravitreal aflibercept injection 2.0 mg prior to vitrectomy. Visual acuity in the eyes of the control group significantly improved only at 6 months after vitrectomy surgery. This is likely to be associated with the incidence of transient vitreous hemorrhage in the postoperative period. Thus, the percentage of eyes with transient vitreous hemorrhage in the early postoperative period was 90% for the control group, which was significantly larger than for the groups preoperatively treated with intravitreal aflibercept injection 1.0 mg (17%; p1-2=0,0003) and intravitreal aflibercept injection 2.0 mg (18%; p1-3=0,0004). In addition, the percentage of eyes with transient vitreous hemorrhage at 2 months was significantly smaller for group 3, but not for group 2, compared to the control group (7.4% versus 40%; p = 0.017). There was no significant difference in percentage of eyes with transient vitreous hemorrhage at 6 months between the control group and aflibercept groups (p1-2=0.45, p1-3= 0.17). Ruban [26] found that the major risk factors for neovascular glaucoma after vitrectomy in patients with PDR were the absence of preoperative panretinal laser photocoagulation; gender; age; type of diabetes; absence of anti-VEGF agent used as adjunct therapy during or after surgery; vitreous hemorrhage; tractional retinal detachment; HbA1 level; iris rubeosis; and neovascular glaucoma in the fellow eye. In the current study, the incidence of iris rubeosis over 6 months after vitrectomy for PDR was significantly larger for the control group than for the groups preoperatively treated with intravitreal aflibercept injection 1.0 mg or intravitreal aflibercept injection 2.0 mg (p = 0.02). Conclusion Therefore, our modified staged method of surgical treatment (involving intravitreal aflibercept injection 1-mg prior to vitrectomy) for PDR enabled an improvement in visual acuity at 2 months and 6 months after surgery; a reduction in the percentage of eyes developing transient vitreous hemorrhage in the early postoperative period; and a 16% reduction in the risk of the development of iris rubeosis in the late period (6 months) after surgery compared to vitrectomy without the use of preoperative anti-VEGF treatment.
References 1.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012; 60(5): 428-31. 2.Schachat AP, Oyakawa RT, Michels RG, Rice TA. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983; 90(5): 522-30. 3.Novak MA, Rice TA, Michels RG. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984 Dec;91(12):1485-9. 4.Blankenship GW. Management of vitreous cavity hemorrhage following pars plana vitrectomy for diabetic retinopathy. Ophthalmology. 1986 Jan;93(1):39-44. 5.Tolentino FI, Cajita VN, Gancayco T, Skates S. Vitreous hemorrhage after closed vitrectomy for proliferative diabetic retinopathy. Ophthalmology. 1989 Oct;96(10):1495-500. 6.Neely KA, Scroggs MW, McCuen BW II. Peripheral retinal cryotherapy for postvitrectomy diabetic vitreous hemorrhage in phakic eyes. Am J Ophthalmol. 1998 Jul;126(1):82-90. 7.Yeh PT, Yang CM, Yang CH. Cryotherapy of the anterior retina and sclerotomy sites in diabetic vitrectomy to prevent recurrent vitreous hemorrhage: an ultrasound biomicroscopy study. Ophthalmology. 2005 Dec;112(12):2095-102. 8.Yang CM, Yeh PT, Yang CH. Intravitreal long-acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology. 2007 Apr;114(4):710-5. 9.Wang DY, Zhao XY, Zhang WF. Perioperative anti-vascular endothelial growth factor agents treatment in patients undergoing vitrectomy for complicated proliferative diabetic retinopathy: a network meta-analysis. Sci Rep. 2020 Nov 3;10(1):18880. 10.Pasyechnikova NV, Naumenko VO, Korol AR, Zadorozhnyy OS, Kustryn TB, Henrich PB. Intravitreal ranibizumab for the treatment of choroidal neovascularizations associated with pathologic myopia: a prospective study. Ophthalmologica. 2015;233(1):2-7. 11.Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. Jul-Aug 2006;26(6):699-700. 12.Yeoh J, Williams C, Allen P. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin Exp Ophthalmol. 2008 Jul;36(5):449-54. 13.Rizzo S, Genovesi-Ebert F, Di Bartolo E. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol. 2008 Jun;246(6):837-42. 14.El-Batarny AM. Intravitreal bevacizumab as an adjunctive therapy before diabetic vitrectomy. Clin Ophthalmol. 2008 Dec;2(4):709-16. 15.Ahmadieh H, Shoeibi N, Entezari M. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009 Oct;116(10):1943-8. 16.Yeh PT, Yang CM, Lin YC. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina. 2009 Jun;29(6):768-74. 17.Ponomarchuk Vira S, Umanets MM, Velychko LM. [Vitreous VEGF levels and features of vitrectomy after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy]. In: [Proceedings of the National Conference on Current Issues of Ophthalmology]. September 22-23, 2021. Mykolaiv, Ukraine. Ukrainian. 18.Ponomarchuk Vira S, Velychko LM, Umanets MM. Vitreous VEGF levels among patients with proliferative diabetic retinopathy depending on the general clinical status and ocular status. J Ophthalmol (Ukraine). 2021;4: 19-25. 19.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002 Dec;29(6 Suppl 16):10-4. 20.Korol A, Kustryn T, Zadorozhnyy O. Comparison of Efficacy of Intravitreal Ranibizumab and Aflibercept in Eyes with Myopic Choroidal Neovascularization: 24-Month Follow-Up. J Ocul Pharmacol Ther. 2020 Mar;36(2):122-125. 21.Gupta A, Bansal R, Gupta V. Six-month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab. Int Ophthalmol. 2012 Apr;32(2):135-44. 22.Hu L, Chen Q, Du Z. Evaluation of vitrectomy combined preoperative intravitreal ranibizumab and postoperative intravitreal triamcinolone acetonide for proliferative diabetic retinopathy. Int Ophthalmol. 2021 May;41(5):1635-1642. 23.Lo WR, Kim SJ, Aaberg TM Sr. Visual outcomes and incidence of recurrent vitreous hemorrhage after vitrectomy in diabetic eyes pretreated with bevacizumab (avastin). Retina. Jul-Aug 2009;29(7):926-31. 24.Velazquez JC, Aleman I, Rush SW. Bevacizumab before Diabetic Vitrectomy: A Clinical Trial Assessing 3 Dosing Amounts. Ophthalmol Retina. 2018 Oct;2(10):1010-1020. 25.Zhao LQ, Zhu H, Zhao PQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol. 2011 Sep;95(9):1216-22. 26.Ruban AM. [Risk factors for neovascular glaucoma after miniinvasive vitrectomy for proliferative diabetic retinopathy]. Medychna informatika ta inzheneriia. 2014;3:51-6. Ukrainian.
Acknowledgement: The authors thank O.I. Dragomiretska for her assistance in statistic analysis. Author Contributions: Vira S. Ponomarchuk: conducting intravitreal injections, data collection and analysis, writing the text; A.R. Korol: idea to use 1 mg aflibercept, editing the text; M.M.Umanets: concept and design of the study, surgical interventions, and editing. Disclosures: The authors declare no conflict of interest. Source of support: There are no external sources of funding.
|