J.ophthalmol.(Ukraine).2022;1:24-29.
|
http://doi.org/10.31288/oftalmolzh202212429 Received: 06 October 2021; Published on-line: 15 March 2022
Percentage expression of neutrophil activation marker in the peripheral blood of patients with dry eye disease plus type 2 diabetes Zhmud T. M. 1, Velychko L. N. 2, Drozhzhyna G. I. 2, Bogdanova O. V. 2 1 National Pirogov Memorial Medical University, Vinnytsya; Vinnytsya (Ukraine) 2 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: gtatyana@email.ua TO CITE THIS ARTICLE: Zhmud T. M., Velychko L. N., Drozhzhyna G. I., Bogdanova O. V. Percentage expression of neutrophil activation marker in the peripheral blood of patients with dry eye disease plus type 2 diabetes. J.ophthalmol.(Ukraine).2022;1:24-9. http://doi.org/10.31288/oftalmolzh202212429 Background: Dry eye disease (DED) is a disorder of the anterior segment of the eye which is common in patients with type 2 diabetes mellitus (T2DM). It has been reported that symptoms of DED were observed in 60-70% of patients with T2DM. Given that inflammation has a key role in the progression of both T2DM and DED, we believe it is reasonable to study the role of neutrophils in this process. Purpose: To assess the percentage expression of CD15, a neutrophil activation marker, in the peripheral blood of patients with both DED and T2DM. Material and Methods: Forty-six patients (92 eyes; mean age, 54.0 ± 8.0 years) with both DED and T2DM were included in this study. There were 19 (40%) women and 27 (60 %) men. Mean diabetes duration was 8.0 ± 6.6 years. All patients had well-compensated diabetes. In addition to a routine examination of the eye, Schirmer I test and tear film break-up time (TBUT) test, they had their corneal fluorescein staining (CFS) scored using the Oxford schema, severities of dry eye graded according to the DEWS II classification and Ocular Surface Disease Index (OSDI) obtained. An immunohistocytochemical study was employed to assess the expression of CD15, a neutrophil activation marker, in peripheral blood cells of patients. Results: The mean percentage expression of CD15, a neutrophil activation marker, in the peripheral blood, was 46.7% for 14 patients with T2DM plus DED and punctuate keratopathy, and 28.5% for 32 patients with T2DM plus DED and intact cornea (р=0.0001). There was a mild negative correlation between the expression of CD15 and the Schirmer score (r = -0.32; р = 0.032), and between the former and the TBUT score (r = -0.34; р = 0.019). Keywords: type 2 diabetes mellitus, dry eye disease, neutrophil activation marker
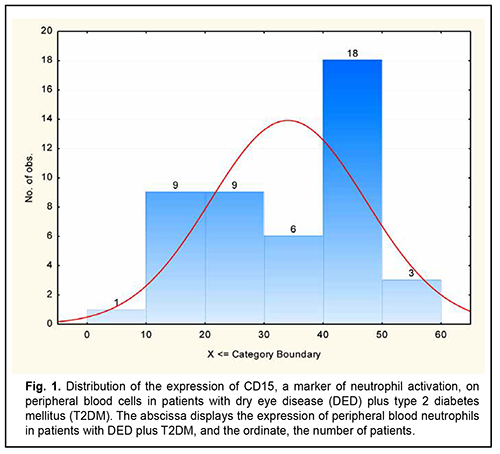
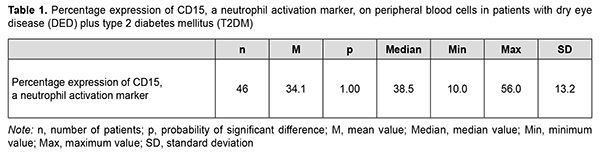
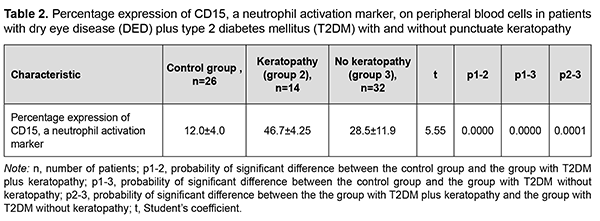
The global prevalence of type 2 diabetes mellitus (T2DM) has increased considerably, and ocular diabetic complications (like dry eye disease (DED), diabetic keratopathy (DK), and diabetic retinopathy (DR)) may result in serious visual impairment and blindness [1, 2]. DED is a disorder of the anterior segment of the eye which is common in patients with T2DM. It has been reported that symptoms of DED were observed in 54-70% of patients with T2DM [3, 4]. Chronic hyperglycemia is the core causative mechanism for DK, and causes the release of various cytokines, chemokines, cell adhesion molecules and other proapoptotic genes [5]. Although it is known that the inflammatory component plays a key role in DED [6], the role of neutrophils in the pathogenesis of the disease is still poorly understood. Increased activity of matrix metalloproteinases [7, 8], neutrophil elastase [8], myeloperoxidase [7, 8] and Neutrophil Extracellular Traps (NETs) [7, 9, 10] are pathognomonic signs of the presence of neutrophils in the tear fluid of patients with inflammatory ocular surface disorders like DED. The involvement of neutrophils increases with increased severity of DED and associated conditions. It has been reported that NETs play a role in the pathogenesis of severe DED [11, 10], and that the medications for NETs may be helpful in severe DED. Some experimental and clinical material has been accumulated that allows us to consider the activation of neutrophils as a reaction that, in the presence of inflammation and under certain conditions, may result in their uncontrolled cytotoxicity as well as tissue destruction. The mechanisms are, however, still to be elucidated. Various proteins are known to be located on the surface of immunocompetent cells, and it is the identification of these proteins that will make it possible to assess the function of these cells. These proteins are named CD markers, have a role as receptors involved in cellular interaction, and are components of signal transduction pathways. The official listing of determinants has identified over 350 individual and unique markers, but we need detailed studies on some of them for understanding pathological processes, particularly those in diabetes. Kuryltsiv and colleagues [12] assessed the expression of CD15, a neutrophil activation marker, and CD95, a marker of apoptosis, in patients with intermediate uveitis, and hypothesized that neutrophils may be responsible for tissue damage in these patients. Given that inflammation has a key role in the progression of both T2DM and DED, we believe it is reasonable to study the role of neutrophils in this process. The purpose of this study was to assess the percentage expression of CD15, a neutrophil activation marker, in the peripheral blood of patients with both DED and T2DM. Material and Methods Forty-six patients (92 eyes; mean age, 54.0 ± 8.0 years) with both DED and T2DM were included in this study. There were 19 (40%) women and 27 (60 %) men. Mean diabetes duration was 8.0 ± 6.6 years. All patients had well-compensated diabetes, with a mean glucose level of 7.2 ± 0.8 mmol/L. The control group was composed of 26 healthy individuals (mean age, 63.76 ± 6.67 years). Exclusion criteria were connective tissue disorders, history of eye surgery, current use of any ocular medication, and a 7-day or shorter history of acute inflammation of the ocular surface. In addition to a routine examination of the eye, Schirmer I test and tear film break-up time (TBUT) test, patients had their corneal fluorescein staining (CFS) scored using the Oxford schema, severities of dry eye graded according to the DEWS II classification and Ocular Surface Disease Index (OSDI) obtained [13, 14]. The study followed the ethical standards stated in the Declaration of Helsinki, European Convention on Human Rights and Biomedicine, relevant provisions of the WHO, and Order of the Ministry of Health of Ukraine No.281 of November 1, 2000. Patients with T2DM were divided into two groups, group 1 (dry eye with punctuate keratopathy; 14 patients) and group 2 (dry eye with intact cornea; 32 patients), based on the state of the cornea. Most patients of group 1 had a CFS grade of 1 or 2, whereas patients of group 2 had a CFS grade of 0, using the Oxford schema [13]. Severities of dry eye graded according to the DEWS II classification. Patients of group 1 had moderate (grade 2) DED, whereas patients of group 2, mild (grade 1) DED [14]. Patients of group 2 (T2DM+DED with intact cornea) had an OSDI score corresponding to a dry eye of mild severity, whereas most patients of group 1 (T2DM+DED with punctuate keratopathy), an OSDI score corresponding to a dry eye of moderate severity [13]. An immunohistocytochemical study using monoclonal antibodies (the peroxidase anti-peroxidase method) [15] was employed to assess the expression of CD15, a neutrophil activation marker, in 46 patients with both DED and T2DM and 26 individuals of the control group. CD15, a neutrophil activation marker, is expressed on mature granulocytes, monocytes, and normal and leukemic myelomonocytes. In the course of immunohistocytochemical study, clear neutrophil suspension was obtained and supplemented with monoclonal antibodies specific for CD15. One hundred free neutrophils were evaluated under the miscroscope, and the percentage of CD15-positive neutrophils was calculated. CD15-positive neutrophils exhibit a dark brown rim of the cytoplasm [15]. Statistica 8 (StatSoft, Tulsa, OK, USA) software was used for statistical analysis. Data are represented as the mean (M) ± standard error of mean (m) or standard deviation (SD). The Kolmogorov-Smirnov test was used to check for the normality of data distribution. The Student's t-test for independent and dependent samples was applied. Spearman's correlation coefficient (r) was used to assess correlation between the expression of CD15 on neutrophils and the Schirmer score, and between the former and the TBUT score. Results The distribution of CD15 expression on peripheral blood neutrophils of patients with both DED and T2DM had two maximum values: neutrophil CD15 expression in 39% of the patients with both DED and T2DM was 10% to 30%, and in another 39% of the patients, 40% to 50% (Fig. 1, Table 1). The mean expression of CD15, a neutrophil activation marker, in the peripheral blood, was 46.7% for T2DM patients with punctuate keratopathy, and 28.5% for T2DM patients with intact cornea (the difference was statistically significant), and 12.0±4.0 % for the controls (р=0.0001; Table 2).
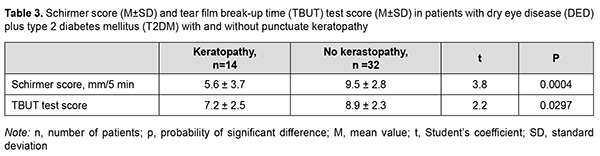
Table 3 shows Schirmer test and TBUT test scores for DED patients with versus without punctuate keratopathy. All patients showed reduced tear production, and mean Schirmer score was 5.6 ± 3.7 mm in 5 minutes for those with punctuate keratopathy versus 9.5 ± 2.8 mm in 5 minutes for those with intact cornea (р=0.0004; Table 3). Mean tear film break-up time was 7.2 ± 2.5 sec for patients with punctuate keratopathy versus 8.9 ± 2.3 sec for those with intact cornea (р=0.0297) (Table 3).
There was a negative correlation between the expression of CD15 on neutrophils and the Schirmer score (r = -0.32; р = 0.032), and between the expression of CD15 on neutrophils and the TBUT score (r = -0.34; р = 0.019). An OSDI score > 15 was present in all patients, indicating the presence of dry eye symptoms. In addition, mean OSDI score was 17.0 ± 2.0, corresponding to a dry eye of mild severity, for patients of group 2 (T2DM plus DED) versus 28.0 ± 2.0, corresponding to a dry eye of moderate severity, for patients of group 1 (T2DM plus punctuate keratopathy). We found that the percentage expression of CD15 on neutrophils did not depend on patient’s gender, type of treatment (blood sugar reducing tablets (р=0.40) or insulin injections (р=0.39)), age (р=0.23), blood sugar level (р=0.19), or diabetes duration (р=0.92). Discussion Recent studies have demonstrated that circulating neutrophils may perform various functions like phagocytosis щк protein synthesis, and be involved in oxidative metabolism. Until recently, there were few studies on phenotypic differences in neutrophils, and it was the detection of highly specific markers of neutrophils that enabled to divide this subpopulation of cells into subgroups. In addition, although neutrophils were believed to have only an antimicrobial function, studies have demonstrated that they have various functional responses that go beyond just elimination of microorganisms [16]. Various functional responses of neutrophils are induced by activation of transcription and changes in the expression patterns and activity of the superficial molecules. These phenotypic changes are usually observed only in the subgroup of neutrophils exhibiting heterogeneous phenotypes. Aside from a protective function, neutrophils have been shown to have an activated state, which, under certain conditions, may result in additional tissue damage. The release of cytotoxic products from neutrophil granules leads to tissue disintegration [17]. Enough material has been accumulated that allows us to consider the activation of neutrophils as a reaction that, under certain conditions, may result in their uncontrolled cytotoxicity as well as tissue destruction [17]. It should be noted that activated neutrophils circulating in blood are very different in behavior from those in tissues. Wedmore and Williams [18] demonstrated that activated neutrophils have a disruptive effect on the integrity of the epithelium and cause increased permeability and dissociation of endothelial cell-to-cell junction proteins. The interaction of circulating neutrophils with activated endothelium is a central phenomenon in inflammation, and involves various molecular events associated with neutrophil adhesion and migration as well as impaired endothelial permeability. Neutrophils must be stimulated by inflammatory mediators and by adhesion surfaces for the initiation of cytotoxic activity [19]. Excessive neutrophil-endothelial cell adhesion may result in endothelial damage by proteases and oxidants [20]. Endothelial dysfunction and increased levels of anti-inflammatory cytokines may result in leikostasis and increased interaction of leukocytes with epithelial cells. Subsequently to neutrophil activation, there is the release of elastase and myeloperoxidase from neutrophil granule, leading to corneal damage. It has been demonstrated in animal models and in human disease that the prevention of or reduction in leukocyte-endothelial cell adhesion frequently resulted in significantly reduced microcirculatory dysfunction and parenchymal cell dysfunction [4]. In addition, it has been hypothesized that ocular surface infiltration with T cells (particularly, Th1 і Th17) is implicated in damage to ocular surface epithelial cells and plays a major role in the development of dry eye disease [21, 22]. We have previously assessed the activity of oxidative reductive enzymes and glutathione levels in the cornea, conjunctiva and tear fluid in experimental streptozotocin-induced diabetes. We found that the activity of oxidative reductive enzymes in the cornea of animals with acute conjunctivitis in the presence of streptozotocin-induced diabetes was substantially decreased compared to animals with conjunctivitis induced in the absence of experimental diabetes. In addition, under the above conditions, the activity of oxidoreductases (lactate dehydrogenases, malate dehydrogenases and glucose-6- phosphate dehydrogenases) in the tear fluid is significantly increased, indicating impaired stability of cell membrane structures [23, 24]. Moreover, it was noted that, the development of inflammation in the conjunctiva under hyperglycemic conditions results in a more severe impairment in glutathione redox status (particularly, a reduction in reduced glutathione and increase in oxidized glutathione) than in conjunctival changes in non-diabetic animals [25-27]. The results obtained indicated an impairment of the antioxidant system and damage to lysosomal membranes of epithelial cells in experimental diabetes, with these system and membranes playing a major role in homeostasis of the ocular surface. We were the first to assess the expression of CD15, a neutrophil activation marker, in peripheral blood cells of patients with both DED and T2DM. Given the fact that activated neutrophils have a role in the development of changes (i.e., triggering of immune inflammation) in the ocular surface, it would be reasonable to consider integrating anti-neutrophil therapy (aiming to reduce the release of inflammatory mediators) use as an adjunct in T2DM management. The use of anti-inflammatory (anti-neutrophil) therapy will reduce capillary permeability (and, consequently, reduce the exudative component of inflammation); stabilize lysosomal membranes and reduce lysosomal enzyme secretion (thus reducing tissue alteration); and inhibit the synthesis of inflammatory mediators [18, 19]. The differences that we found in the expression of CD15, a neutrophil activation marker, in peripheral blood cells, between the two groups (the group of patients with DED plus T2DM and the presence of punctuate keratopathy, and the group of those without punctuate keratopathy), suppose that CD15 may be a subclinical predecessor of immune inflammation in the cornea. The data obtained suppose that activated neutrophils may trigger immune inflammation resulting in corneal damage in patients with T2DM due to the release of pro-inflammatory cytokines. Understanding of the mechanisms and the role of neutrophils in the pathological processes in the cornea in patients with DED plus T2DM suggests the need for the use of anti-leukocyte agents to prevent keratopathy progression. Therefore, further studies are required (a) to determine changes in the phenotype of neutrophils in DED for improved understanding of their role in abnormal regulation of the ocular surface and (b) to assess their role as biomarkers of the disease. Conclusion First, we found that, the mean expression of CD15, a neutrophil activation marker, in the peripheral blood, was 46.7% for patients with T2DM plus DED and punctuate keratopathy, and 28.5% for patients with T2DM plus DED and intact cornea (р=0.0001). Second, there was a negative correlation between the expression of CD15, a neutrophil activation marker, and the Schirmer score (r = -0.32; р = 0.032), and between the former and the tear film break-up time (TBUT) score (r = -0.34; р = 0.019).
References 1.Zhao H, He Y, Ren Y-R, Chen B-H. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol. 2019 Dec 18;12(12):1939-50. 2.Threatt J, Williamson JF, Huynh K, Davis RM, Hermayer K. Ocular disease, knowledge and technology applications in patients with diabetes. Am J Med Sci. 2013 Apr;345(4):266-70. 3.Zhmud ТМ, Malachkova NV, Andrushkova OO, Hrizhymalska КY. Meibomian gland dysfunction and dry eye disease symptoms in patients with type 2 diabetes mellitus. Reports of Morphology. 2019;25(4): 51-5. 4.Cowburn A S, Condiffe AM, Farahi N, et al. Advances in neutrophil biology: Clinical implications. Chest. 2008 Sep;134(3):606-612. 5.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018 Jan;16(1):45-57. 6.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017 Jul;15(3):276-283. 7.Arafat SN, Suelves AM, Spurr-Michaud S, et al. Neutrophil collagenase, gelatinase, and myeloperoxidase in tears of patients with Stevens-Johnson Syndrome and Ocular Cicatricial Pemphigoid. Ophthalmology. 2014 Jan;121(1):79-87. 8.Arafat SN, Robert M-C, Abud T, et al. Elevated neutrophil elastase in tears of ocular graft-versus-host disease patients. Am J Ophthalmol. – 2017 Apr;176:46-52.. 9.Tibrewal S, Sarkar J, Jassim SH, et al. Tear fluid extracellular DNA: diagnostic and therapeutic implications in dry eye disease. Invest Ophthalmol Vis Sci. 2013 Dec 11;54(13):8051-6. 10.Sonawane S, Khanolkar V, Namavari A, Chaudhary S, Gandhi S, Tibrewal S, et al. Ocular surface extracellular DNA and nuclease activity imbalance: A new paradigm for inflammation in dry eye disease. Invest Ophthalmol Vis Sci. 2012;53(13):8253–63. 11.Postnikoff CK, Huisingh C, McGwin G, Nichols KK. Leukocyte distribution in the open eye tears of normal and dry eye subjects. Curr Eye Res. 2018 Oct;43(10):1253-1259. 12.Kuryltsiv NB, Zborovska OV, Velychko LM, Bogdanova OV. [Role of CD markers in the development of uveal inflammation]. Zdobutky klinichnoi i eksperymentalnoi medytsyny. 20121;2:96-101. Ukrainian. 13.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003 Oct;22(7):640-50. 14.Lemp MA, Baudouin C, Baum J, Dogru M, Foulks GN, Kinoshita S et al. The definition and classification of dry eye disease: report of the definition and classification. Subcommittee of the International Dry Eye Workshop. Ocul Surf. 2007;5 (2):75–92. 15.Gluzman DF, Skliarenko LM, Nadgornaia VA, Kriachok IA. [Immunocytochemistry in tumor diagnosis]. Kyiv: Morion;2003. Russian. 16.Potapnev MP, Hushchyna LM, Moroz LА. [Human neutrophils subpopulations and functions heterogeneity in norm and pathology]. Immunologiia. 2019; 40 (5): 84–96. Russian. 17.Galkin AA, Demidova VS. [Neutrophils and systemic inflammatory response syndrome]. Rany i ranevyie infektsii. Zhurnal imeni prof. B.M. Kostiuchenka. 2015;2:25-32. Russian. 18.Wedmore CV, Williams TJ. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646-50. 19.Segel G, Halterman M, Lichtman M. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011 Mar;89(3):359-72. 20.Panes J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol. 1999 Feb;126(3):537-50. 21.Stevenson W, Chauhan SK, Dana R. Dry eye disease: An immune-mediated ocular surface disorder. Arch Ophthalmol. 2012 Jan;130(1):90-100. 22.Perez VL, Pflugfelder SC, Zhang S, Shojaei A. Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016 Apr;14(2):207-15. 23.Zhmud TM. [Intensity of redox processes in the cornea in experimental keratitis in the presence of diabetes]. Oftalmologiia. 2015;2(2):202-10. Russian. 24.Zhmud TM, Leus NF, Drozhzhyna GI. [Changes in the activity of oxidative enzymes in the cornea and tear in keratitis in the presence of diabetes]. Oftalmologiia. Vostochnaia Ievropa. 2018;8(2):221-32. Russian. 25.Drozhzhyna GI, Zhmud TM. [Experimental prerequisites for improving glutathione level in the ocular mucosa and tear in acute conjunctivitis under conditions of hyperglycemia]. Oftalmol Zh. 2014;6:66-71. Russian. 26.Zhmud TM. [Investigating redox potentials of the thiol system of the cornea in experimental keratitis in the presence of diabetes]. Oftalmol Zh. 2015;6:46-9. Ukrainian. 27.Zhmud TM. [State of the thiol system of the cornea in animal models of acute conjunctivitis and streptozotocin-induced diabetes]. Oftalmologiia. Vostochnaia Ievropa. 2015;4(27):42-8. Russian.
Acknowledgement: The authors thank O.I. Dragomiretska for her assistance in statistic analysis. Conflict of Interest Statement: The authors declare that there is no actual or potential conflict of interest (financial, personal, professional and other interests) that could affect the opinion on the subject or materials described and discussed in this manuscript.
|