J.ophthalmol.(Ukraine).2022;1:11-19.
|
http://doi.org/10.31288/oftalmolzh202211119 Received: 03 November 2021; Published on-line: 15 March 2022 Ocular hemodynamics in patients with posterior uveitis entities N. I. Khramenko, N. V. Konovalova SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: khramenkon@gmail.com TO CITE THIS ARTICLE: Khramenko N. I., Konovalova N. V. Ocular hemodynamics in patients with posterior uveitis entities. J.ophthalmol.(Ukraine).2022;1:11-9. http://doi.org/10.31288/oftalmolzh202211119 Background: In spite of recent advances in ophthalmology, the diagnosis and treatment of uveitis is still a challenge. Purpose: To assess ocular hemodynamics in patients with primary and recurrent posterior uveitis (focal and disseminated chorioretinitis) using ophthalmic rheography. Material and Methods: One hundred and eighteen patients (mean age ± standard deviation, 37.2 ± 1.5 years) with posterior uveitis were included in the study. The median duration of recurrent posterior uveitis was 2920 days (range, 1080 to 5110 days). The etiology of chorioretinitis was not established in all patients of the study. The age-matched control group was composed of 16 healthy volunteers (32 eyes). Patients and controls underwent ophthalmic rheography (ORG) with Reocom, the computerized rheography apparatus (Kharkiv, Ukraine). ORG included measurements of ocular pulse blood filling (expressed as RQ, ‰ rheographic coefficient) and vascular tone (expressed as alpha/T percentage index), and volumetric pulse blood filling rate (expressed as Ohm/s). Results: In primary focal and disseminated chorioretinitis, inflammation was characterized by increased ocular circulation, which was manifested by (a) 20% increased ocular pulse blood filling (both for unilateral and bilateral inflammation); (b) 1.4-times and twice, respectively, increased volumetric pulse blood filling rate and (c) 12.5% and 33.3%, respectively, increased vascular tone in large-caliber vessels and small-caliber vessels. Ocular hemodynamics was increased in patients with unilateral recurrent focal, bilateral recurrent focal and bilateral recurrent disseminated chorioretinitis in the period of recurrence; particularly, ocular pulse blood filling was 17% increased, and volumetric pulse blood filling rate, 1.6 times increased. In addition, vascular tone in large-caliber vessels was increased. The period of remission in patients with focal chorioretinitis was characterized by normal ocular pulse blood filling, whereas in those with recurrent disseminated chorioretinitis, by 20% decreased ocular pulse blood filling compared to the norm. Volumetric pulse blood filling rate in the period or remission was 1.4-1.5-times increased in patients with focal chorioretinitis, but not in those with disseminated chorioretinitis. Conclusion: Ocular pulse blood filling, volumetric pulse blood filling rate, and vascular tone in large-caliber and small-caliber vessels were increased in patients with primary posterior uveitis and those with recurrent posterior uveitis in the period of recurrence, both for unilateral and bilateral inflammation. In patients with recurrent posterior uveitis, the period of remission was characterized by a wide range of changes, from normal values to mild deficits, in ocular pulse blood filling, as well as increased vascular tone in vessels, which requires adequate anti-ischemic treatment in the period between recurrences. Keywords: posterior uveitis entities, ophthalmic rheography, ocular hemodynamics
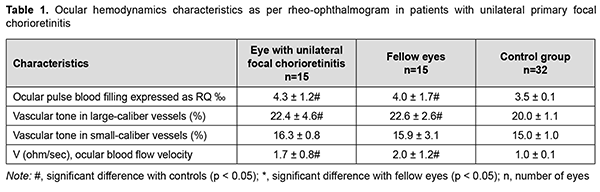
Introduction In spite of recent advances in ophthalmology, the diagnosis and treatment of uveitis is still a challenge. The issue is important since the disease may result in fast decline in visual acuity and cause severe complications and early visual disability. The incidence and prevalence of uveitis differ based on age, anatomic location of the inflammatory process (anterior, intermediate, posterior uveitis, or panuveitis), gender, histopathology (granulomatous or non-granulomatous), type of inflammatory process (acute, chronic, or recurrent), and etiology (infectious, non-infectious, neoplastic or traumatic). In addition, the prevalence differs among the geographical regions [1]. A systematic review with meta-analysis was conducted by García-Aparicio and colleagues [2]. Medline, Embase, and Cochrane Library were searched from inception to January 2019. A total of 49 studies were included and critically appraised. For prevalence studies, data ranged from 9 to 730 cases per 100,000. For incidence studies, the meta-analysis yielded a pooled incidence of 50.45 per 100.000. The meta-regression showed the geographic region as an important explanatory factor of the heterogeneity between studies. It was concluded that population-based estimates of the epidemiology of uveitis vary widely, owing to methodologies employed, definitions of uveitis and geographical regions; the representativeness and generalizability of many epidemiological studies of uveitis are limited [2]. Zhang and colleagues [3] aimed to evaluate the epidemiology of infectious uveitis/scleritis employing a large US national medical claims database. The mean annual rates of any uveitis/scleritis diagnosis within the Optum population having 15 months of continuous enrollment from 2007–2015 were 124.3 (incidence) and 316.4 (prevalence) per 100,000 persons. Uveitis is estimated to cause 10–15% of blindness in the United States, affecting a wide demographic range of patients [4]. The disease is mostly caused by endogenous factors (infections and general medical conditions) [5], with almost 150 general medical conditions representing potential causes. In the developed world, infectious uveitis can comprise up to 20% of all uveitis cases, and toxoplasmosis and herpetic infection predominate [1]. In the developing world, infectious uveitis may account for up to 30–50% of all cases of uveitis [1, 6], with the most common etiologies including toxoplasmosis, tuberculosis, onchocerchiasis, and cysticercosis. However, in many cases, uveitis remains idiopathic in spite of an extensive systemic evaluation [1]. Uveitis can occur at any age, but mostly affects young adults of working age [6, 7]. Ocular tissues become involved in inflammation when an infectious agent overcomes general and local defense mechanisms. The disease may spread to the eye exogenously (through the epithelium and along conjunctival and corneal nerve endings) or endogenously (hematogenously or neutrally, via sensory, motor and sympathetic nerve fibers) [8]. Chronic and recurrent inflammation frequently results in complications, leading to visual impairment or blindness, and, consequently, reduced quality of life [9, 10]. Posterior uveitis is inflammation of the posterior uveal tract (involves the retina and choroid). According to the International Uveitis Study Group and Standardization of Uveitis Nomenclature working group classification, posterior uveitis includes focal, multifocal or diffuse choroiditis, chorioretinitis, retinochoroiditis, retinitis, and neuroretinitis [11, 12]. Posterior uveitis is difficult to diagnose, has a prolonged course, is not uncommonly chronic or recurrent and bilateral, and not uncommonly causes various complications, loss of capacity for work for a long period of time, and early visual disability. Etiological and differential diagnosis of chorioretinitis is challenging, partly due to polymorphic clinical picture and the absence of pathognomonic symptoms. Advanced non-invasive high-technology modalities like comprehensive ultrasound and optical coherence tomography (OCT) are commonly used for diagnosis of ocular disorders. Recent advances in research into the immunological mechanisms of uveitis are associated with the advent of new opportunities for the analysis of cellular and molecular immunogenetic mechanisms, and are highlighted as being of interest for clarifying the immunological aspects of the pathogenesis of chorioretinitis [13, 14]. The ocular circulation system is also important for effective implementation of the immune response. The unique anatomic features of the eye, particularly, the presence of the two large vascular systems (the retinal and uveal vascular systems) as well as the blood-retinal barrier, to a large extent, determine the features of inflammation. The development of effective and safe treatment methods requires better understanding of the molecular, cellular, tissue and systemic mechanisms of the disease, and which deficiencies in the regulation of immune, nervous, endocrine and vascular systems are pathogenetic for uveitis. Predicting changes in ocular hemodynamics for different periods of the disease is important for predicting complications and outcome [12]. The purpose of the study was to assess ocular hemodynamics in patients with primary and recurrent posterior uveitis (focal and disseminated chorioretinitis) using ophthalmic rheography. Material and Methods One hundred and eighteen patients who were examined and treated for posterior uveitis on inpatient or outpatient basis at the Department of Ocular Inflammatory Disease, Filatov Institute of Eye Disease and Tissue Therapy, were included in the study. Of these, 33 patients had primary uveitis of 3 months or less duration (including 19 patients (23 eyes) with focal chorioretinitis and 14 patients (18 eyes) with disseminated chorioretinitis); 45 patients, a remission episode of recurrent uveitis (including 32 patients (49 eyes) with focal chorioretinitis and 15 patients (30 eyes) with disseminated chorioretinitis); and 36 patients, a recurrent episode of uveitis (including 25 patients (36 eyes) with focal chorioretinitis and 13 patients (26 eyes) with disseminated chorioretinitis). The average patient age was 37.2±1.5 years (mean ± standard deviation). The median duration of recurrent posterior uveitis was 2920 days (range, 1080 to 5110 days). The etiology of chorioretinitis was not established in all patients of the study. The control group was composed of 16 volunteers (32 eyes) of matched age without ocular disease or general medical condition. Patients underwent assessment of visual acuity, ophthalmoscopy, biomicroscopy, perimetry, and examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance. In addition, patients and controls underwent ophthalmic rheography (ORG) with Reocom, the computerized rheography apparatus (KhAI-Medika, Kharkiv, Ukraine). ORG included measurements of ocular pulse blood filling (expressed as RQ, ‰ rheographic coefficient) and vascular tone (expressed as alpha/T percentage index), and volumetric pulse blood filling rate (expressed as Ohm/s). In addition, the vascular tone in large-caliber vessels and the vascular tone in small-caliber vessels were calculated on the bases of low-frequency and high-frequency components, respectively, of differential rheograms. Analysis was performed using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. The Kolmogorov-Smirnov test was performed to determine normality of data. Normally distributed data are presented as mean and standard deviation (SD) and non-normal data are presented as median (Me) and inter-quartile range (IQR). The Student's t test was used to determine significant differences between two normally distributed samples. Mann-Whitney U test was used for the comparison of two samples when the underlying distributions were not normal. The correlation was calculated by Spearman’s rank correlation. Results In patients with unilateral primary focal chorioretinitis, ocular pulse blood filling (expressed as RQ, ‰ rheographic coefficient) (a) in the affected eye was 4.3 ± 1.2‰, which was 22.8% and significantly (p = 0.002) higher than in controls (Table 1), and (b) in the fellow eye was 14.3% and significantly (p = 0.02) higher than in controls. There was, however, no significant difference in ocular pulse blood filling between the affected eye and the fellow eye (Table 1). In addition, the vascular tone in large-caliber vessels in the affected eye and fellow eye was 12.5% and significantly (p = 0.002) higher than in controls. There was, however, no significant difference in the vascular tone in small-caliber vessels between patients with unilateral primary focal chorioretinitis and controls. Moreover, in patients with unilateral primary focal chorioretinitis, volumetric pulse blood filling rate in the affected eye and fellow eye was 1.7-times and twice (p = 0.002), respectively, higher than in controls (Table 1).
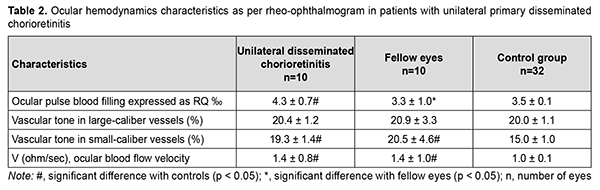
In patients with unilateral primary disseminated chorioretinitis, ocular pulse blood filling (a) in the affected eye was 22.8% and significantly (p = 0.002) higher than in controls and (b) in the fellow eye was 30.3% and significantly (p = 0.009) higher than in controls. In addition, the vascular tone in small-caliber vessels in the affected eye and fellow eye was 33% and significantly (p = 0.002) higher in patients with unilateral disseminated chorioretinitis than in controls. Moreover, in patients with unilateral disseminated chorioretinitis, volumetric pulse blood filling rate in both eyes was 1.4 times (p = 0.003) higher than in controls.
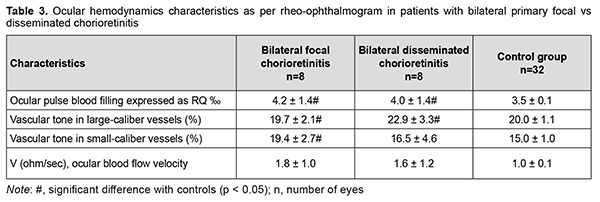
In patients with bilateral primary focal chorioretinitis and in those with bilateral primary disseminated chorioretinitis, ocular pulse blood filling was 20% (p = 0.003) and 14.3% (p = 0.02), respectively, higher, whereas, volumetric pulse blood filling rate, 1.6-times and 1.8-times (p = 0.001), respectively, higher, compared to controls (Table 3).
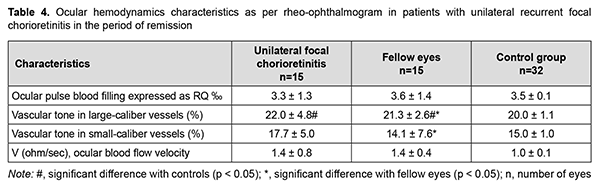
Therefore, in primary focal and disseminated chorioretinitis, inflammation was characterized by increased ocular circulation, which was manifested by (a) 20% increased ocular pulse blood filling (both for unilateral and bilateral inflammation); (b) 1.4-times and twice, respectively, increased volumetric pulse blood filling rate and (c) 12.5% and 33.3%, respectively, increased vascular tone in large-caliber vessels and small-caliber vessels. In patients with recurrent focal chorioretinitis, ocular hemodynamics was examined during a remission episode as well as during a recurrence episode. This was important due to a direct correlation between the ocular pulse blood filling and the period (a recurrence or remission episode) of the disease (Spearmen r = 0.32; p < 0.05) and between volumetric pulse blood filling rate and the period (a recurrence or remission episode) of the disease (Spearmen r = 0.28; p < 0.05). There was no significant difference in the ocular pulse blood filling between patients with recurrent unilateral focal chorioretinitis (the affected eye or fellow eye) in the period of remission and controls (Table 4). However, vascular tone in large-caliber vessels and small-caliber vessels was 10% (p = 0.04) and 18% (p = 0.003), respectively, increased, and volumetric pulse blood filling rate, 1.4-times (p = 0.003) increased, in patients with recurrent unilateral focal chorioretinitis (the affected eye or fellow eye) in the period of remission compared with controls (Table 4).
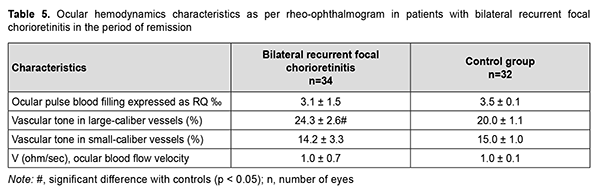
There was no significant difference in the ocular pulse blood filling between patients with recurrent bilateral focal chorioretinitis in the period of remission and (a) controls (Table 5) or (b) patients with recurrent unilateral focal chorioretinitis in the period of remission (Table 4). Vascular tone in large-caliber vessels was 20% (p = 0.001) increased in these eyes of patients compared with controls.
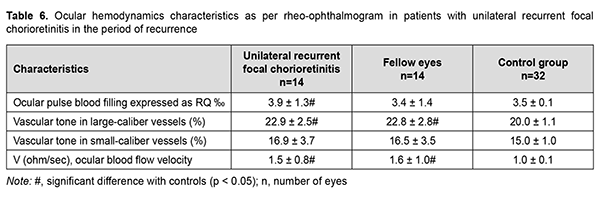
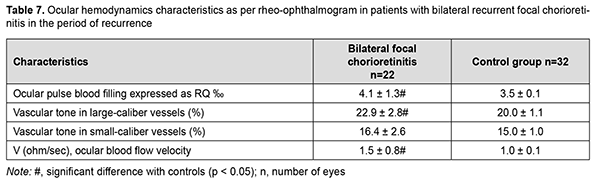
Therefore, the ocular pulse blood filling in the affected and fellow eyes of patients with recurrent unilateral focal chorioretinitis in the period of remission was not different from normals, and this was true also for patients with recurrent bilateral focal chorioretinitis in the period of remission. Vascular tone in large-caliber vessels was 10% but not significantly increased in unilateral chorioretinitis, and 20% and significantly increased in bilateral chorioretinitis (p = 0.01) compared with controls. Volumetric pulse blood filling rate was 1.4 times increased in eyes with unilateral chorioretinitis compared with controls. In recurrent focal unilateral chorioretinitis, inflammation was characterized by increased ocular circulation, which was manifested by (a) 11.4% (p = 0.04) increased ocular pulse blood filling; (b) 14.5% (p = 0.001) increased vascular tone in large-caliber vessels and (c) 1.5-times (p = 0.001) increased volumetric pulse blood filling rate compared with controls. In the fellow eye of patients with recurrent focal unilateral chorioretinitis, RQ was not significantly different from that of controls, but vascular tone in large-caliber vessels was 14.5% (p = 0.001) increased, and volumetric pulse blood filling rate, 1.6 times (p = 0.001) increased compared with controls. In patients with recurrent focal bilateral chorioretinitis in the period of recurrence, ocular pulse blood filling was 17.1% (p = 0.005) increased; vascular tone in large-caliber vessels, 14.5% (p = 0.001) increased; and volumetric pulse blood filling rate, 1.5 times (p = 0.001) increased, compared with controls. It is noteworthy that there was no significant difference in RQ between patients with recurrent focal unilateral chorioretinitis in the period of recurrence and those with recurrent focal bilateral chorioretinitis in the period of recurrence, with mean RQ being 4.03 ± 1.3‰. Therefore, ocular hemodynamics was increased in patients with recurrent focal unilateral chorioretinitis in the period of recurrence; particularly, ocular pulse blood filling was 14.3% increased, and volumetric pulse blood filling rate, 1.6 times increased. In addition, similar to that in the period of remission, vascular tone in large-caliber vessels was 14.5% increased.
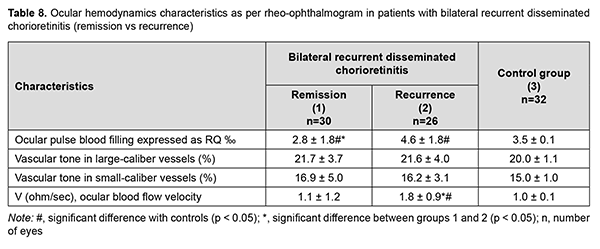
There were no patients with recurrent disseminated unilateral chorioretinitis in the study cohort. In patients with recurrent disseminated bilateral chorioretinitis, ocular pulse blood filling in the period of remission was 20% (p = 0.04) decreased, and in the period of recurrence, 31.4% (p = 0.001) increased, compared to the norm. In general, ocular pulse blood filling in these patients in the period of recurrence was 64% higher than in the period of remission (Table 7).
Volumetric pulse blood filling rate increased 1.8-fold (p = 0.009) in the period of recurrence (Table 8). No statistically significant difference was found in ocular pulse blood filling between patients with recurrent focal chorioretinitis and those with recurrent disseminated chorioretinitis in the period of recurrence (p = 0.06). The mean value for this characteristic in the period of recurrence was 4.1±1.6 %, which was 17% higher than the norm (p = 0.02).
Therefore, in primary focal and disseminated chorioretinitis, inflammation was characterized by increased ocular circulation, which was manifested by (a) 20% increased ocular pulse blood filling (both for unilateral and bilateral inflammation); (b) 1.4-times and twice, respectively, increased volumetric pulse blood filling rate and (c) 12.5% and 33.3%, respectively, increased vascular tone in large-caliber vessels and small-caliber vessels. In patients with recurrent focal chorioretinitis and those with recurrent disseminated chorioretinitis in the period of recurrence, (a) ocular pulse blood filling was 17% increased; (b) volumetric pulse blood filling rate, 1.6-times increased and (c) vascular tone in large-caliber vessels and small-caliber vessels, 14.5% and 20%, respectively, increased. The period of remission in patients with focal chorioretinitis was characterized by normal ocular pulse blood filling, whereas in those with recurrent disseminated chorioretinitis, by 20% decreased ocular pulse blood filling compared to the norm. Volumetric pulse blood filling rate was 1.4-1.5-times increased in patients with focal chorioretinitis in the period or remission, but not in those with recurrent disseminated chorioretinitis in the period or remission. Discussion Uveitis is characterized by a wide variety of pathological processes, e.g., inflammation, increased vascular permeability and occlusion, local ischemia and cell alteration by inflammatory mediators. Severe complications like macular edema or neovascularization can occur, leading to reduced vision. In addition, some inflammatory lesions are difficult to differentiate from vascular lesions. Early detection and monitoring of these changes can be critical for adequate management of patients with uveitis. Studies on ocular hemodynamics may improve our knowledge on the pathophysiology and natural course of the disease, and help guiding uveitis specialist’s decision making [15]. One should remember that inflammation is a complex protective and adjustment response of an organism to injury or pathogenic action, this response being manifested by the three major interrelated processes, alteration, exudation and proliferation. Inflammation is characterized by several local and general signs. External signs can be clinically determined and have been known for long as the four cardinal signs of Celcus and the fifth added later by Galen. These signs are: (1) redness (Latin: rubor; erythema) from arterial hyperemia(arterial hyperemia is observed in initial inflammation and is gradually changed by venous hyperema, with the affected site found intensive red and even bluish should the circulation become restricted or arrested); (2) swelling (Latin: tumor; edema) due to increased permeability of small capillaries and venules and exudate release to the intercellular space, with a subsequent increase in volume of inflamed tissue; (3) heat (Latin: calor; a local increase in temperature) due to increased arterial blood flow to inflammatory foci as a result of increased metabolism therein; (4) pain (Latin: dolor) accompanies inflammation and is mainly determined by two mechanisms: mechanical compression of sensory nerve terminals during effusion and a humoral mechanism, with inflammatory mediators acting on the receptor apparatus at the focus of inflammation, leading to pain response; and (5) disturbance of function (Latin: functio laesa); function of inflamed organs may be decreased, distorted, increased or totally lost [16, 17]. Therefore, the vascular system plays an important role in inflammation. Due to anatomic features of the ocular vascular system, there are few non-invasive techniques for assessing ocular hemodynamics characteristics in various pathological processes, particularly in uveitis. Fluorescein angiography (FA) and spectral-domain optical coherence tomography (SD-OCT) remain the most commonly used tools to detect uveitic complications. However, standard FA requires an invasive procedure, does not reliably provide capillary-level resolution, and needs subjective assessment that does not correlate with OCT anatomic findings in many cases [18, 19]. Ultra-widefield FA is helpful for detecting signs of peripheral vasculitis, capillary nonperfusion, and vascular leakage in uveitis. The parameters used to quantify the extent of peripheral vasculitis, capillary nonperfusion, and vascular leakage in uveitis are foveal avascular zone area and macular leakage, peripheral diffuse capillary leakage and ischemia, peripheral vasculitis, and leakage from neovascularization [20]. The choroidal imaging has received considerable attention in the past decade due to the advancements in imaging techniques, especially OCT including enhanced depth imaging and swept-source (SS) OCT. OCT including enhanced depth imaging enables measurements of choroidal thickness, choroidal volume and choroidal vascularity index (CVI), the latter representing the contribution of luminal area of choroidal blood vessels to the total choroidal thickness [21]. We have previously found that, in eyes with acute focal chorioretinitis, at the area of chorioretinal scarring, neurosensory retinal thickness was 5.5% to 37% thinner at the site of inflammation at the fovea, whereas parafoveal retinal thickness as well as peripapillary retinal thickness was 3% to 53% thicker due to increased swelling, compared to controls. In addition, minimum choroidal thickness was 348 ± 38 μm, and maximum choroidal thickness, 735 ± 33 μm, in eyes with acute focal chorioretinitis, which was 24% thicker and 133% thicker, respectively, compared to controls, and these differences were significant (p = 0.0001) [22, 23]. With an advent of OCT angiography (OCTA), morphofunctional assessment of the vascular plexuses has become possible. While this imaging modality has been used for various ocular conditions, the application OCTA to uveitis conditions remains sparse [25]. It is used in iris vessel dilation seen in various forms of iritis, as a predictive factor for further episodes of inflammation. OCTA can also depict ischemia in the deep plexus layers of the retina. In addition, OCTA can depict neovascularization in granulomatous disease of the retina or choroid not previously depicted with previous imaging methods. While OCTA provides several advancements in the imaging, management and prognosis of uveitis diseases, further studies are required to fully understand its application to these conditions [19]. SS-OCTA imaging can provide improved visualization of blood flow in the choroid and CC due to better depth penetration through the RPE/ Bruch’s membrane (BM) complex and lower sensitivity roll-off when compared to SD-OCT systems. Additionally, acquisition speeds of more than 100 kHz facilitate wide angle imaging, a feature important for diseases like uveitis that frequently manifest clinically significant lesions in the extrafoveal macula. Moving from SD-OCTA to SS-OCTA has made choroidal anatomy more accessible and improved the quality of images for quantitative analysis, but high quality, high resolution images are still difficult to obtain. Quantitative metrics of the retinal vasculature have been developed that describe the length, density, or branching patterns of the vessels and these metrics have been used to define characteristics of the retinal vasculature in healthy and diseased eyes. Due to many possible causes of media opacity in patients with uveitis, these high-quality scans are not always achievable. Future studies with larger sample sizes are certainly warranted to determine the clinical relevance of this type of image analysis to specific forms of posterior uveitis [24]. The application OCTA to uveitis conditions remains sparse. A quantitative analysis of choriocapillaris (CC) flow deficits (FDs) in patients with uveitis found that posterior uveitis patients have significantly larger CC FDs than patients with other forms of uveitis. Retinal venous velocities evaluated by retinal function imager were significantly decreased in eyes with uveitic cystoid macular edema (CME) relative to controls. Decreased venous velocity was correlated with increased central retinal thickness in uveitic eyes [25]. Chudinova and Hokkanen [26] used Doppler ultrasound to study the state of regional hemodynamics for chorioretinitis of different etiology. They found that maximum and minimum blood flow velocities in the posterior short ciliary arteries and maximum and minimum blood flow velocities in the posterior long ciliary arteries in the presence of choroidal proliferative process were significantly decreased compared with the control group [26]. In the current study, ophthalmic rheography was used to assess a similar type of hemodynamics changes in eyes with posterior recurrent uveitis in the period of remission. While using ophthalmic rheography for assessing ocular and brain hemodynamics in eyes with anterior uveitis, we have found that, compared to patients without macular edema (ME), those with ME exhibited a 50% and 22.2% higher ocular pulse blood filling in primary anterior uveitis and in chronic anterior uveitis, respectively. Ocular pulse blood filling of the internal carotid system in patients with recurrent anterior uveitis complicated by ME was 30% higher than in patients with uncomplicated recurrent anterior uveitis [28]. The current ophthalmic rheography study found increased ocular circulation in primary posterior uveitis and recurrent posterior uveitis in the period of recurrence (focal and disseminated chorioretinitis), which was manifested by (a) 17% and 20%, respectively, increased ocular pulse blood filling; (b) 1.4-times and twice, respectively, increased volumetric pulse blood filling rate and (c) 12.5% and 33.3%, respectively, increased vascular tone in large-caliber vessels and small-caliber vessels, both for unilateral and bilateral inflammation, compared with controls. In patients with recurrent posterior uveitis, the period of remission was characterized by a wide range of changes, from normal values to mild deficits, in ocular pulse blood filling, as well as increased vascular tone in vessels, which requires adequate anti-ischemic treatment in the period between recurrences. Further improvement in our knowledge of pathophysiological processes in uveitis conditions is essential in order to determine the site of therapeutic action, reduce complication rate and improve the quality of life of patients.
References 1.Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A Focus on the Epidemiology of Uveitis. Ocul Immunol Inflamm. 2018;26(1):2-16. 2.García-Aparicio Á, García de Yébenes MJ, Otón T, Muñoz-Fernández S. Prevalence and Incidence of Uveitis: A Systematic Review and Meta-analysis. Ophthalmic Epidemiol. 2021 Feb 8:1-8. 3.Zhang Y, Amin S, Lung KI, Seabury S, Rao N, Toy BC. Incidence, prevalence, and risk factors of infectious uveitis and scleritis in the United States: A claims-based analysis. PLoS One. 2020 Aug 25;15(8):e0237995. 4.Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of noninfectious uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016;134(11):1237–45. 5.Foster CS. Diagnosis and Treatment of Uveitis. 2nd ed. Jaypee Brothers Medical Publishers: New Delhi; 2013. 6.Nussenblatt RB, Whitcupp SM. Fundamentals and Clinical Practice. 4th ed. Philadelphia, PA: Mosby-Elsevier; 2010. 7.Lee JH, Mi H, Lim R, Ho SL, Lim WK, Teoh SC, et al. Ocular Autoimmune Systemic Inflammatory Infectious Study—Report 3: Posterior and Panuveitis. Ocul Immunol Inflamm. 2019;27(1):89–98. 8.Miserocchi E. Review on the world-wide epidemiology of uveitis. Eur J Ophthalmol. Sep-Oct 2013;23(5):705-17. 9.Katsnelson LA, Tankovskii VE. Uveitis: Clinical Features and Treatment. 2nd Updated and Revised Ed. Moscow: 4th Voenizdat Branch; 2003. Russian. 10.Venkataraman A, Rathinam SR, Venkataraman A. A pre- and post-treatment evaluation of vision-related quality of life in uveitis. Indian J Оphthalmol. Jul-Aug 2008;56(4):307-12. 11.Bloch-Michel E, Nussenblatt R.B. International uveitis study group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987 Feb 15;103(2):234-5. 12.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005 Sep;140(3): 509–16. 13.Forrester JV, Kuffova JV, Forrester L, Dick AD. Autoimmunity, autoinflammation, and infection in uveitis. Am J Ophthalmol. 2018 May;189:77-85. 14.Avetisov SE, Sdobnikova SV, Surnina ZV, et al. [Idiopathic uveitis entities: new possibilities in the diagnosis (preliminary report)]. Point of View. East - West. 2018;4:8-9. Russian. 15.Agarwal A, Hassan M, Afridi R, et al. The role of optical coherence tomography angiography in the management of uveitis. Int Ophthalmol Clin. 2016;56(4):1–24. 16.Ushakova TM, Derezina TN, Poloziuk ON. [Pathological physiology: A manual for veterinary students]. Part 1. Persianovskii: Donskoi GAU; 2017. Russian. 17.Ado AD, editor. [Pathological physiology]. Moscow: Triada-Kh;2000. Russian. 18.Kim AY, Rodger DC, Shahidzadeh A, Chu Z, Koulisis N, Burkemper B, et al. Quantifying Retinal Microvascular Changes in Uveitis Using Spectral-Domain Optical Coherence Tomography Angiography. Am J Ophthalmol. 2016Nov;171:101-112. 19.Pichi F, Sarraf D, Arepalli S, Lowder CY, Cunningham ET Jr, Neri P, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res. 2017 Jul;59:178-201 20.Karampelas M, Sim DA, Chu C, Carreno E, Keane PA, Zarranz-Ventura J, et al. Quantitative analysis of peripheral vasculitis, ischemia, and vascular leakage in uveitis using ultra-widefield fluorescein angiography. Am J Ophthalmol. 2015 Jun;159(6):1161-1168.e1. 21.Singh SR, Rasheed MA, Goud A, Sahoo NK, Vupparaboina KK, Chhablani J. Diurnal variation in subfoveal and peripapillary choroidal vascularity index in healthy eyes. Indian J Ophthalmol. 2019 Oct;67(10):1667-1672. 22.Konovalova NV, Khramenko NI, Shaibi A, Ivanitska OV, Naritsyna NI. [Optical coherence tomography study of the sensory retina and choroid in patients with uveitis]. Oftalmol Zh. 2014;3:34-41. Russian. 23.Konovalova NV, Khramenko NI, Shaibi A. [Optical coherence tomography study of the sensory retina and choroid in patients with uveitis]. Point of View. East - West. 2014;1:200-3. Russian. 24.Chu Z, Weinstein JE, Wang RK, Pepple KL. Quantitative Analysis of the Choriocapillaris in Uveitis Using En Face Swept-Source Optical Coherence Tomography Angiography. Am J Ophthalmol. 2020 Oct;218:17-27. 25.Feng X, Kedhar S, Bhoomibunchoo C. Retinal blood flow velocity in patients with active uveitis using the retinal function imager. Chin Med J (Engl). 2013;126(10):1944-7. 2013;126(10):1944-7. 26.Chudinova OV, Hokkanen VM. [Current possibilities of chorioretinites diagnostics]. Oftalmologiia. 2012;9(1):67-72. https://doi.org/10.18008/1816-5095-2012-1-67-72. 27.Khramenko NI, Konovalova NV. [Findings of ocular and brain hemodynamics in patients with anterior uveitis complicated by macular edema]. J Ophthalmol (Ukraine). 2020;4:С. 14-22.
Conflict of Interest Statement: The authors declare no conflicts of interest that could affect their opinion regarding the subject or materials described and discussed in this manuscript.
|