J.ophthalmol.(Ukraine).2022;1:3-10.
|
http://doi.org/10.31288/oftalmolzh20221310 Received: 12 November 2021; Published on-line: 15 March 2022 Angiopoietins and prediction of vitreous hemorrhage in type 2 diabetes patients with diabetic retinopathy S. O. Rykov, S. Iu. Mogilevskyy, S. S. Lytvynenko, S. V. Ziablitsev 1 Shupyk National Healthcare University of Ukraine; Kyiv (Ukraine) 2 Bogomolets National Medical University; Kyiv (Ukraine) E-mail: sergey.mogilevskyy@gmail.com TO CITE THIS ARTICLE: Rykov S. O., Mogilevskyy S. Iu., Lytvynenko S. S., Ziablitsev S. V. Angiopoietins and prediction of vitreous hemorrhage in type 2 diabetes patients with diabetic retinopathy. J.ophthalmol.(Ukraine).2022;1:3-10. http://doi.org/10.31288/oftalmolzh20221310 Background: Various types of vitreoretinal surgery are used for the treatment of severe diabetic retinopathy (DR), with vitreous hemorrhage being a common postoperative complication. Impaired angiopoietin system function in type 2 diabetes mellitus (T2DM) patients with DR plays a role in retinal vascular damage and may determine the development of vitreous hemorrhage, e.g., after vitrectomy. Purpose: To identify the effects of angiopoietins on and their prognostic value in the development of vitreous hemorrhage after advanced vitreoretinal surgery in T2DM patients with DR. Material and Methods: The study included 118 T2DM patients (118 eyes) with DR. These included patients with mild nonproliferative DR (NPDR; Group 1; n = 28), moderate or severe NPDR (Group 2; n = 49), and proliferative DR (PDR; Group 3; n = 41). They underwent a 25-G closed subtotal vitrectomy (CSTV) with panretinal laser photocoagulation. Either a mixture of perfluoropropane (18% C3F8) and air was used to perform tamponade of the vitreous cavity or balance salt solution (BSS plus) was left in the vitreous cavity. Vitreous samples were obtained during vitrectomy, and Ang1 and Ang2 concentrations and their ratio for these samples were measured by enzyme-linked immunosorbent assay. Models were developed using the EZR version 1.54 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing; Vienna, Austria). Results: Vitreous hemorrhage developed in 33.1% of patients with T2DM by three months after vitrectomy for DR, and was directly associated with increased levels of Ang1 and Ang2 in the vitreous (p < 0.005). After adjustment for DR severity stage, the risk of postoperative vitreous hemorrhage in patients with mild NPDR was associated with an increased level of Ang2 in the vitreous (OR, 1.95; 95% CI, 1.06-3.59 per 100 pg/ml), with an optimal cut-off point of 1246 pg/ml (р = 0.003). The risk of postoperative vitreous hemorrhage in patients with severe NPDR and PDR was associated with the level of Ang2 as well as the Ang2/ Ang1 ratio in the vitreous. The study identified cut-off vitreous levels of Ang2 of (a) 2806 pg/mL for moderate or severe NPDR (AUC=0.84; 95% CI, 0.71-0.93; p < 0.001) and (b) 4610 pg/mL for PDR (AUC=0.71; 95% CI, 0.55-0.84; p = 0.013). Conclusion: The results obtained demonstrated that Ang2 accumulation in the vitreous resulted in an increased risk of postoperative vitreous hemorrhage, and was of high prognostic significance. Keywords: closed subtotal vitrectomy, postoperative vitreous hemorrhage, angiopoietins, diabetic retinopathy, type 2 diabetes mellitus
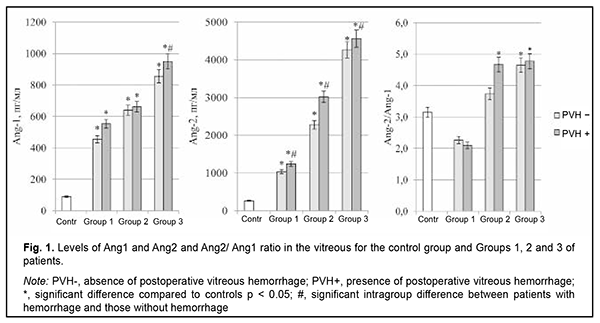
Introduction Globally, the estimated number of people with diabetes increased from 108 million in 1980 to 422 million in 2014 [1]. Diabetes is the most common endocrine disease, and, globally, is the fourth most common cause of death among noncommunicable diseases after cardiovascular disease, cancer and respiratory diseases [2]. The global diabetes prevalence in 20–79 year olds in 2021 was estimated to be 10.5% (536.6 million people), rising to 12.2% (783.2 million) in 2045 [3]. Type 2 diabetes mellitus (T2DM) accounts for around 90% of all cases of diabetes [1], and the number of individuals with T2DM has been increasing especially rapidly. Diabetic retinopathy (DR) is a common complication of T2DM and a significant cause of visual impairment and blindness. The global prevalence of DR, for the period 2015 to 2019 was 27.0% [4]. Lesions and occlusion of retinal vessels result in the development of retinal edema, which increases hypoxic injury, causes damage to neurosensory cells, and contributes to intraocular and retinal hemorrhage [5]. Wang and colleagues [6] analyzed the causes of vitreous hemorrhage in the 40-59 years age group, and the results showed that 43.3% were possibly due to proliferative diabetic retinopathy [6]. Vitreous hemorrhage significantly affects the state of the eye after vitreoretinal surgery and hampers regenerative processes [7, 8]. Postoperative recurrent vitreous hemorrhage developed in 21.6-25.7% of eyes in patients with DR who underwent sutureless vitrectomy with 23-(23G) or 25-gauge (25G) narrow-gauge systems [9]. Angiopoietins are a family of growth factors that are ligands for the tyrosine kinase receptor (TIE2), whose role involves regulation of endothelial function and microvascular permeability; in addition, TIE2 plays a part in angiogenesis and vascular remodeling [10]. Ang1 is a secreted factor encoding a protein of 498 amino acids and there is approximately a 60% sequence homology between Ang1 and Ang2. Ang1 regulates maturation and decreases permeability of neovascular vessels and inhibits fibrosis [11], whereas Ang2 has negative effects that depend on the expression of vascular endothelial growth factor (VEGF). Ang2 affects endothelial cell junctions in vessels, which increases vascular permeability, leading to the escape of blood plasma and proteins into surrounding tissues [12]. Ang2 is believed to be a negative regulator of TIE2 activity, and, in the presence of hypoxia and ischemia, mediates inactivation of TIE2, which can destabilize the vascular network and increase sensitivity to VEGF and other pro-inflammatory cytokines [13]. Therefore, impaired angiopoietin system function in T2DM patients with DR plays a role in retinal vascular damage and may determine the development of hemorrhagic complications like vitreous hemorrhage after vitrectomy. The purpose of the study was to identify the effects of angiopoietins on and their prognostic value in the development of vitreous hemorrhage after advanced vitreoretinal surgery in T2DM patients with DR. Material and Methods The study included 118 T2DM patients (118 eyes) with DR. These included patients with mild nonproliferative DR (NPDR; Group 1; n=28), moderate or severe NPDR (Group 2; n=49), and proliferative DR (PDR; Group 3; n=41). There were 52 (44.1%) men and 66 (55.9%) women. Patient age ranged from 44 to 84 years (mean age, 63.8 ± 8.9 years). T2DM duration in the study population ranged from 4 to 45 years. Particularly, T2DM duration in Group 1 ranged from 4 to 21 years (median, 10 years; QІ-QІІІ, 5.5-14.5 years); Group 2, from 4 to 45 years (median, 15 years; QІ-QІІІ, 10-20 years); and Group 3, from 10 to 35 years (median, 20 years; QІ-QІІІ, 15-20.25 years). The study design and protocol were approved by the ethics committee of Shupyk National Healthcare University of Ukraine, adhered to the tenets of the Declaration of Helsinki of 1964 with its further amendments and European Convention on Human Rights and Biomedicine, and were compliant with the relevant legal requirements of Ukraine. Informed consent was obtained from all patients. CSTV was performed in patients of groups 1 and 2 with a progressive reduction in central and peripheral vision; visual field defects in the central and paracentral regions; changes in the quality of vision in the presence of (a) NPDR with refractive macular edema or vitreomacular syndrome with the presence of tangential tractions resulting from incomplete detachment of the posterior hyaloid membrane and (b) diabetic maculopathy (DMP) with an epimacular membrane. Indications for CSTV in patients of group 3 were progressive PDR with refractive macular edema; fibrovascular membranes; axial and tangential tractions on the retina with imminent tractional retinal detachment; or presence of vitreous, preretinal and/or subhyaloid hemorrhage. Exclusion criteria were severe PDR and the presence of tractional retinal detachment or massive hemorrhage in the course of CSTV during removal of the fibrovascular tissue which required a silicone oil tamponade of the vitreous cavity. Patients underwent an eye examination which included visual acuity assessment, tonometry, perimetry, keratometry, refractometry, slit lamp biomicroscopy, gonioscopy, ophthalmoscopy with Volk Super Field lens and Goldmann three-mirror lens (Volk Optical, Mentor, OH) and fundus photography (the Early Treatment Diabetic Retinopathy Study (ETDRS) seven standard fields) with the fundus camera TRC-NW7SF (Topcon, Tokyo, Japan) [14]. In addition, they underwent spectral domain optical coherence tomography (SD-OCT; Copernicus REVO, Optopol Technology Sp, zo.o, Zawiercie, Poland; scan programs, Retina 3D and Retina Raster) and OCT (Retina Angio mode, 6 x 6 mm). The intraocular pressure (IOP) was normal, ranging from 16 to 25 mmHg (mean IOP, 19.5 ± 1.25 mmHg). A 25-G CSTV and laser treatment (panretinal laser photocoagulation; PLPC) were performed by the same team of surgeons. Either a mixture of perfluoropropane (18% C3F8) and air was used to perform tamponade of the vitreous cavity or balance salt solution (BSS plus) was left in the vitreous cavity. Vitreous samples were obtained during vitrectomy, and concentrations of Ang1 and Ang2 in these samples were measured by enzyme-linked immunosorbent assay (ELISA) (RnD systems, Minneapolis, MN, USA) in accordance with the manufacturer’s protocol. The Ang1/ Ang2 ratio was calculated. Statistical analyses were performed with EZR version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing; version 4.0.3, R Foundation for Statistical Computing, Vienna, Austria) [15]. The Shapiro–Wilk test was used to assess the normality of the data in analyses of distributions of quantitative variables. The quantitative data (Ang1 and Ang2 levels in the vitreous) were presented as median (Me) and interquartile range (Q1-Q3) values, since the distributions of these data were not normal distributions. The Mann-Whitney test was applied for comparisons of two groups. For comparisons of more than two groups, Kruskal-Wallis test with Dunn's post hoc analyses was used. A stepwise forward/backward regression analysis was performed (with a P < 0.10 and a P > 0.20 used as cutoff points for forward selection and backward deletion) to identify a minimum set of independent variables that are predictive of postoperative vitreous hemorrhage for a multivariate regression model, taking in account the Ang1 and Ang2 levels in the vitreous as well as their ratio. Predictive performance of the models was assessed for discrimination with receiver operator characteristic (ROC) curves. The area under the curve (AUC) was calculated for each ROC curve with 95% confidence intervals (CI) and its associated sensitivity and specificity were calculated. The model was considered adequate if the AUC was significantly different from 0.5. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated to assess the relative contribution of each independent variable. Two-tailed tests were used with a significance level of 0.05. Results By three months after surgery, vitreous hemorrhage developed in 33.1% of the study patients (28.6% of patients of Group 1; 30.6% of patients of Group 2; and 39.0% of patients of Group 3). Vitreous levels of Ang1 and Ang2 were significantly higher (p < 0.001) in patients of all treatment groups compared to controls (Fig. 1).
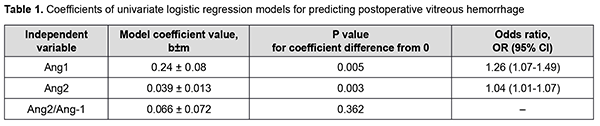
There was a statistically significant increase in the vitreous levels of Ang1 and Ang2 with an increase in DR severity stage. Vitreous levels of Ang2 were higher than those of Ang1 in Groups 2 and 3; this was reflected in increased Ang2/Ang1 ratios for patients of these groups. In addition, vitreous levels of Ang1 were higher in patients with postoperative vitreous hemorrhage, and this difference was statistically significant for patients of Group 3 only. Moreover, vitreous levels of Ang2 were higher in patients with postoperative vitreous hemorrhage, and this difference was statistically significant for patients of all treatment groups (Fig. 1). Table 1 presents the results of the analysis of the relationship between Ang1 and Ang2 levels in the vitreous and postoperative vitreous hemorrhage.
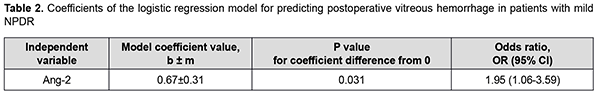
There was an increase (p = 0.005) in the risk of postoperative vitreous hemorrhage with an increase in the vitreous level of Ang1 (OR, 1.26; 95% CI, 1.07-1.49 per 100 pg/ml). In addition, there was an increase (p = 0.003) in the risk of postoperative vitreous hemorrhage with an increase in the vitreous level of Ang2 (OR, 1.04; 95% CI, 1.01-1.07 per 100 pg/ml). At the second stage of our analysis, we considered the relationships between vitreous levels of Ang1 and Ang2 and postoperative vitreous hemorrhage separately for each DR severity stage. The vitreous level of Ang2 was selected as the only independent variable significantly associated with the risk of postoperative vitreous hemorrhage for patients with mild NPDR (Table 2).
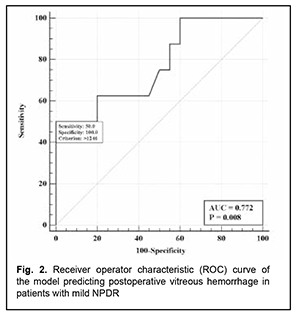
The logistic regression model built using the selected independent variable was found to be adequate (χ2=8.7; p = 0.003). The area under the curve (AUC) was 0.77 (95% CI, 0.58-0.91), indicating a moderate association between the vitreous level of Ang2 and postoperative vitreous hemorrhage after CSTV for patients with mild NPDR. Fig. 2 shows the ROC curve for the model predicting postoperative vitreous hemorrhage for patients with mild NPDR.
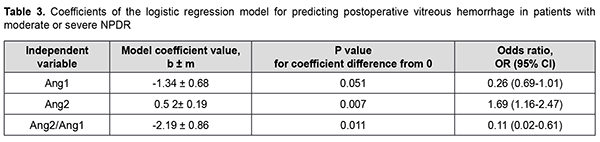
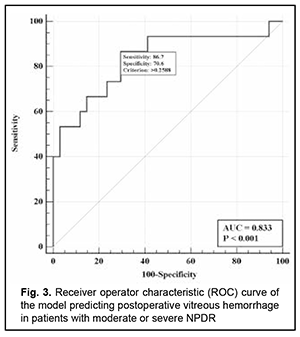
There was an increase (p=0.031) in the risk of postoperative vitreous hemorrhage with an increase in the vitreous level of Ang2 (OR, 1.95; 95% CI, 1.06-3.59 per 100 pg/ml) for patients with mild NPDR. The vitreous level of Ang2 for the optimal cut-off point associated with the Youden index was 1246 pg/ml, with a model sensitivity of 50% (95% CI, 15.7%-84.3%) and specificity of 100% (95% CI, 83.2%-100%). Three independent variables (vitreous levels of Ang1 and Ang2 and their ratio) were selected as independent variables significantly associated with the risk of postoperative vitreous hemorrhage for patients with moderate or severe NPDR (Table 3). The logistic regression model built using the selected independent variables was found to be adequate (χ2=17; p = 0.001). Fig. 3 shows the ROC curve for the model predicting postoperative vitreous hemorrhage for patients with moderate or severe NPDR. The AUC was 0.83 (95% CI, 0.70-0.92), indicating a strong association between the selected independent variables and postoperative vitreous hemorrhage after CSTV.
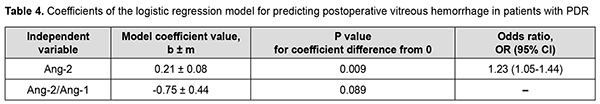
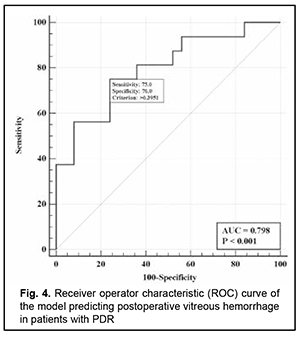
There was an increase (p=0.007) in the risk of postoperative vitreous hemorrhage with an increase in the vitreous level of Ang2 (OR, 1.69; 95% CI, 1.16-2.47 per 100 pg/ml, after adjusting for other risk factors) for patients with moderate or severe NPDR. In addition, the risk of postoperative vitreous hemorrhage was associated (p=0.011) with the Ang2/Ang1 ratio (OR, 0.11; 95% CI, 0.02-0.61 per unit, after adjusting for other risk factors) for patients with moderate or severe NPDR. For the optimal cut-off point associated with the Youden index (Ycrit=0.259), the model sensitivity was 50% (95% CI, 59.5%-98.3%) and specificity, 70.6% (95% CI, 52.5%-84.9%). Our analysis of the risk of postoperative vitreous hemorrhage on the basis of ROC analysis allowed identifying the cut-off value of vitreous level of Ang2 for patients with moderate or severe NPDR. For the vitreous level of Ang2 above 2806 pg/ml, the development of postoperative vitreous hemorrhage was probable with a sensitivity of 60.0% and specificity of 82.4% (AUC=0.84; 95% CI, 0.71-0.93; p < 0.001). Two independent variables (the vitreous level of Ang2 and the ratio of vitreous levels of Ang2 and Ang1) were selected as independent variables significantly associated with the risk of postoperative vitreous hemorrhage for patients with PDR. These variables, along with their corresponding coefficients and odds ratios, are included in Table 4. The logistic regression model built using the selected independent variables was found to be adequate (χ2=13; p = 0.002). Fig. 4 shows the ROC curve for the model predicting postoperative vitreous hemorrhage for patients with PDR. The area under the curve (AUC) was 0.80 (95% CI, 0.64-0.91), indicating a moderate association between the selected independent variables and postoperative vitreous hemorrhage after CSTV.
There was an increase (p=0.009) in the risk of postoperative vitreous hemorrhage with an increase in the vitreous level of Ang2 (OR, 1.23; 95% CI, 1.05-1.44 per 100 pg/ml, after adjusting for other risk factors) for patients with PDR. For the optimal cut-off point associated with the Youden index (Ycrit=0.395), the model sensitivity was 75% (95% CI, 47.6%-92.7%) and specificity, 76% (95% CI, 54.9%-90.6%). Our analysis of the risk of postoperative vitreous hemorrhage on the basis of ROC analysis allowed identifying the cut-off value of vitreous level of Ang2 for patients with PDR. For the vitreous level of Ang2 above 4610 pg/ml, the development of postoperative vitreous hemorrhage was probable with a sensitivity of 55.0% and specificity of 76.0% (AUC=0.71; 95% CI, 0.55-0.84; p = 0.013). Discussion Therefore, our study demonstrated an increase in vitreous levels of Ang1 and, especially, Ang2, in DR and T2DM, which depended on DR severity stage. Our findings are in agreement with those reported previously [11, 13, 16, 17]. Thus, Skowerski and colleagues [16] and Raj [17] demonstrated significantly increased Ang2 levels in patients with T2DM in the presence of DR. In the presence of hypoxia and ischemia, increased Ang2 levels destabilize TIE2, which has a pathogenetic role for microangiopathy, as they cause damage to pericytes, detachment of pericytes from the basement membrane, and pericyte migration [18]. It has been reported that increased Ang2 levels facilitate a pathological increase in blood-retinal barrier permeability, which is induced by increased VEGF levels in patients with macular edema in NPDR and T2DM [19]. In a study by Watanabe and co-workers [20], vitreous levels of Ang2 and VEGF were significantly higher in patients with PDR than in control patients. In addition, the vitreous concentration of Ang2 correlated significantly with that of VEGF in eyes with PDR ([correlation coefficient] rho = 0.497, P = 0.001). Simultaneous accumulation of VEGF and Ang2 in the vitreous in PDR was found to be associated with vitreous hemorrhage [21]. Khalaf and colleagues [19] aimed to determine the serum levels of Ang1, Ang2, soluble vascular endothelial Tie2 and VEGF in the serum of T2DM patients having NPDR or PDR. They found that the serum levels of Ang-2 and VEGF were significantly elevated in NPDR groups and PDR group compared to diabetics without retinopathy groups [19]. Concentrations of Ang1 and Ang2 were significantly increased in vitreous samples from PDR compared to controls [22]. Patel and colleagues [23] measured angiopoietin concentrations in vitreous samples from 17 patients with NPDR and clinically significant diabetic macular edema (CSME), 10 patients with PDR, and five patients with macular hole (controls) obtained at pars plana vitrectomy. In NPDR with CSME, Ang1 concentrations were 2002 pg/ml (range 289-5820 pg/ml), and Ang2 concentrations, 4000 pg/ml (range 1341-14329 pg/ml). These findings are in agreement with ours (Fig. 1). The formation of angiopoietins in the retina under hyperglycemic conditions is triggered by the pathophysiological process of hypoxia, which results in a decrease in the numbers of pericytes, epithelial cell destruction, capillary atrophy as well as an increase in capillary loss [24]. In this connection, one should differentiate between the value of increased levels of Ang1and the value of increased levels of Ang2; Ang2 was shown to be a competitive antagonist for Ang1 of the receptor tyrosine kinase TIE2 [25]. TIE2 activation through Ang1 maintains vascular stability and reduces exudation and neovascularization, whereas increased formation of Ang2 under hypoxic conditions inhibits the function of the Ang1/TIE2 axis and affects vascular stability. This has been confirmed in experimental studies. Thus, Yun and colleagues [26] investigated the role of Ang2 in astrocyte loss and vascular leakage in the early streptozotocin-induced diabetic retinopathy and demonstrated that vascular leakage occurred with astrocyte loss in early diabetic mice retina as Ang2 increased. The astrocyte loss and vascular leakage were inhibited by intravitreal injection of Ang2-neutralizing antibody. It was found also that Ang2 can not only destabilize the Ang1/TIE2 axis, but also activate the αvβ5-integrin/GSK-3β/β-catenin pathway, which increases vascular permeability and leads to astrocyte damage in DR. In addition, Ang2 can activate β1-integrin, which additionally reduces endothelial monolayer integrity in a Tie2-independent manner [27]. Moreover, it was found that, in contrast to Ang1, Ang2 cannot activate β1-integrin. β1-integrin binding to Ang2 blocks expression of the membrane-bound TIE2. Ang-2 deficient mice completely lacked hyperglycemia-induced increase in pericyte migration compared with wild-type littermates [28, 29]. The beneficial role of Ang1 has been demonstrated in a diabetic rat model: Ang1 introduction into the vitreous normalized VEGF and intercellular adhesion molecule-1 (ICAM-1) levels and reduced leukocyte adhesion and endothelium and blood-retinal barrier damage [30]. In this connection, the approach of inhibition of Ang2 or the receptors of Ang2 in T2DM is a pathogenetic one. Simultaneous VEGF-A and ANG-2 inhibition by a bispecific domain-exchanged (crossed) monoclonal antibody targeting VEGF-A and ANG-2 was found to reduce vessel lesion number, permeability, retinal edema, and neuron loss more effectively than either agent alone in a spontaneous choroidal neovascularization (CNV) model [31]. Clinical trials have confirmed the efficacy, safety, durability, and superiority of faricimab (a bispecific antibody that binds both VEGF-A and Ang2 without inhibition of Ang1) in patients with diabetic macular edema and age-related macular degeneration [32]. Therefore, Ang 2 not only has negative effects on the Ang1-TIE2 pathway, but also has molecular effects that generally destabilize the endothelium, increase damage to the morphological and functional components of the retina, and contribute to DR progression in T2DM. The fact that their effects are implemented mostly through vascular damage helps explaining our results on the relationship between Ang2 accumulation in the vitreous and the development of postoperative vitreous hemorrhage after CSTV for patients with DR and T2DM. Conclusion First, we found that vitreous hemorrhage developed in 33.1% of patients with T2DM by three months after closed subtotal vitrectomy for DR, and was associated with increased levels of Ang1 and Ang2 in the vitreous. Second, after adjustment for DR severity stage, the risk of the development of postoperative vitreous hemorrhage in patients with mild NPDR was associated with an increased level of Ang2 in the vitreous (OR, 1.95; 95% CI, 1.06-3.59 per 100 pg/ml), with an optimal cut-off point of 1246 pg/ml. Finally, the risk of the development of postoperative vitreous hemorrhage in patients with severe NPDR and PDR was associated with the level of Ang2 as well as the Ang2/ Ang1 ratio in the vitreous. The study identified cut-off vitreous levels of Ang2 of (a) 2806 pg/mL for moderate or severe NPDR (AUC=0.84; 95% CI, 0.71-0.93; p < 0.001) and (b) 4610 pg/mL for PDR (AUC=0.71; 95% CI, 0.55-0.84; p = 0.013).
References 1.World Health Organization. Global Report on Diabetes . Geneva, Switzerland: World Health Organization; 2016. apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Accessed January 2, 2022. 2.World Health Organization. Noncommunicable diseases: key facts. 2021. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Accessed January 2, 2022. 3.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021 Nov 24;109119. 4.Thomas RL, Halim S, Gurudas S, Sivaprasad S, Owens DR. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pract. 2019 Nov;157:107840. 5.Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015 Apr 15;6(3):489-99. 6.Wang CY, Cheang WM, Hwang DK, Lin CH. Vitreous haemorrhage: a population-based study of the incidence and risk factors in Taiwan. Int J Ophthalmol. 2017 Mar 18;10(3):461-466. 7.Marques RE, Sousa DC, Leal I, Faria MY, Marques-Neves C. Complete ILM peeling versus inverted flap technique for macular hole surgery: a meta-analysis. Ophthalmic Surg Lasers Imaging Retina. 2020;51(3):187-A2. 8.Taskintuna I, Elsayed ME, Taskintuna K, Ahmad K, Khandecar R, Schatz P et al. Comparison of outcomes of four different treatment modalities for diabetic vitreous haemorrhage. Sci Rep [Internet]. 2020;10(3674). 9.Ding Y, Yao B, Hang H, Ye Hui. Multiple factors in the prediction of risk of recurrent vitreous haemorrhage after sutureless vitrectomy for non-clearing vitreous haemorrhage in patients with diabetic retinopathy. BMC Ophthalmol. 2020. 10.Hayashi SI, Rakugi H, Morishita R. Insight into the role of angiopoietins in ageing-associated diseases. Cells. 2020;9(12):2636. 11.Khan M, Aziz AA, Shafi NA, Abbas T, Khanani AM. Targeting angiopoietin in retinal vascular diseases: a literature review and summary of clinical trials involving faricimab. Cells. 2020;9(8):1869. 12.Souma T, Thomson BR, Heinen S, Carota IA, Yamaguchi S, Onay T et al. VEPTP determines ANGPT2 activity on TIE2 receptor. Proceedings of the National Academy of Sciences. 2018;115(6);1298-303. 13.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat Rev Drug Discov. 2017;16:635-61. 14.Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs – An Extension of the Modified Airlie House Classification: ETDRS Report Number 10. Ophthalmology. 2020 Apr;127(4S):S99-S119. 15.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-8. 16.Skowerski T, Nabrdalik K, Kwiendacz H, Gumprecht J. Angiopoietin-2 and vascular complications of type 2 diabetes. Clinical diabetology. 2020;9(3):201-204. 17.Raj KK. Angiopoietin 2 in type 2 diabetes mellitus patients and those with complications: an observational comparative study. Int J Adv Med. 2020;7(5):733-6. 18.Geranmayeh MH, Rahbarghazi R, Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun Signal. 2019 Mar 20;17(1):26. 19.Khalaf N, Helmy H, Labib H, Fahmy I, El Hamid MA, Moemen L. Role of Angiopoietins and Tie-2 in Diabetic Retinopathy. Electron Physician. 2017 Aug 25;9(8):5031-5. 20.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005 Mar;139(3):476-81. 21.Keles A, Sonmez K, Erol YO, Ayyıldız SN, Ogus E. Vitreous levels of vascular endothelial growth factor, stromal cell-derived factor-1α, and angiopoietin-like protein 2 in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2021 Jan;259(1):53-60. 22.Yu Y, Zhang J, Zhu R, Zhao R, Chen J, Jin J, Tian Y, Su SB. The Profile of Angiogenic Factors in Vitreous Humor of the Patients with Proliferative Diabetic Retinopathy. Curr Mol Med. 2017 Dec 7;17(4):280-6. 23.Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89(4):480-3. 24.Gupta A, Bhatnagar S. Vasoregression: a shared vascular pathology underlying macrovascular and microvascular pathologies? OMICS. 2015;19(12):733-53. 25.Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. doi: 10.3390/cells8050471 26.Yun JH, Park SW, Kim JH, Park YJ, Cho CH, Kim JH. Angiopoietin 2 induces astrocyte apoptosis via αvβ5-integrin signaling in diabetic retinopathy. Cell Death Dis. 2016 Feb 18;7(2):e2101. 27.Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun. 2015 Jan 30;6:5962. 28.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008 May;49(5):2163-71. 29.Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008 Sep;57(9):2495-502. 30.Cabral T, Mello LG, Lima LH, Polido J, Regatieri CV, Belfort R Jr at al. Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous. 2017;3:31. 31.Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J, Stubenrauch KG, Nogoceke E, Widmer G, Strassburger P, Koss MJ, Klein C, Shima DT, Hartmann G. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2016 Nov 2;8(11):1265-88. 32.Khanani AM, Russell MW, Aziz AA, Danzig CJ, Weng CY, Eichenbaum DA, Singh RP. Angiopoietins as Potential Targets in Management of Retinal Disease. Clin Ophthalmol. 2021 Sep 4;15:3747-55.
Conflict of Interest Statement: The authors declare no conflict of interest. Funding Support: There are no external sources of funding.
|