J.ophthalmol.(Ukraine).2018;5:32-38.
|
https://doi.org/10.31288/oftalmolzh201853238 Received: 23 August 2018; Published on-line: 26 October 2018 Efficacy of complex neuroprotection in glaucomatous optic neuropathy O.V. Guzun, Cand. Sc. (Med.); N.I. Khramenko, Cand. Sc. (Med.); S.B. Slobodianyk, Cand. Sc. (Med.); V.S. Ponomarchuk, Dr. Sc. (Med.), Prof.; O.A. Peretyagin, Cand. Sc. (Med.) SI “Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine”; Odessa (Ukraine) E-mail: olga.v.guzun@gmail.com TO CITE THIS ARTICLE: Guzun OV, Khramenko NI, Slobodianyk SB, Ponomarchuk VS, Peretyagin OA. Efficacy of complex neuroprotection in glaucomatous optic neuropathy. J.ophthalmol.(Ukraine).2018;5:32-38. https://doi.org/10.31288/oftalmolzh201853238 Background. The problem of glaucomatous optic neuropathy is not solved regardless the fact that intraocular pressure is stabilized. To manage the glaucomatous process, effective neuroprotection of the ganglion cells is required. The purpose of the paper was to study the efficacy of complex neuroprotection in patients with glaucomatous optic neuropathy using sequential laser radiation of the papillomacular bundle followed by a six-month vitamin and antioxidant supplement therapy. Material and Methods. We followed up 27 patients (43 eyes) with advanced open angle glaucoma, verified glaucomatous optic neuropathy (GON), and stabilized intraocular pressure (IOP). The patients’ age averaged 63.8 (SD; 6.8). A treatment course included 10 every-day sessions of laser stimulation (LS) using a SM-4.3 unit (wavelength, 650 nm; irradiance, 0.4 mW/cm2; treatment timing, 240 s on the papillomacular bundle) and additional directed laser radiation to the optic disc (OD) using a 90 D lens (treatment timing, 60 s). Thereafter, patients received a vitamin supplement with 1 mg resveratrol: one capsule daily for 6 months. Results. A combined treatment including LS of the optic nerve and the papillomacular bundle followed by a six-month vitamin and antioxidant supplement therapy with resveratrol in patients with glaucomatous optical neuropathy made it possible to increase visual acuity from 0.65 to 0.82 (by 26%), to increase visual system sensitivity with phosphene current threshold decreased from 84.9 μA to 76 μA (by 10%), to improve ocular blood circulation from 2.4 to 2.6‰ (by 8%), to decrease vasospasm from 26.7 to 21.8% (by 18%), significantly increase overall retinal light sensitivity from 1395.0 to 1709.0 dB (by 23%), and to stabilize the glaucomatous process within six months. Analyzing the data obtained after a neuroprotective LS course with a subsequent vitamin antioxidant supplementation therapy with reseratrol (1 mg) revealed an apparent correlation between an increase in overall light sensitivity of the retina and an increase in the blood circulation in the eye (rs=0.71, р<0.01) as well as a decrease in threshold current of visual system sensitivity (rs=-0.84, р<0.01). Conclusions. A combined treatment including LS of the optic nerve and the papillomacular bundle followed by a six-month vitamin and antioxidant supplement therapy with resveratrol (1 mg) in patients with glaucomatous optical neuropathy contributes to: an increase in visual acuity by 26%; an increase in the visual system sensitivity, evidenced by a decrease of phosphene threshold current by 10%; an improvement in ocular blood flow by 8%; a decrease of intraocular vasospasm by 18%; and a significant decrease in overall retinal light sensitivity by 23%. It also supports: an increase in conduction of optic nerve fibers; an improvement of the papillomacular bundle function; and stabilization of the glaucomatous process withing six months. Keywords: glaucomatous optic neuropathy, neuroprotection, , laser stimulation, nutrition therapy
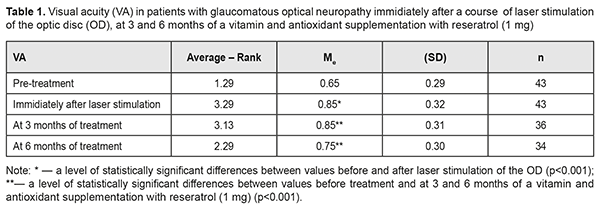
Introduction Glaucoma is a major cause of progressive blindness worldwide. Although the disease is mainly defined as damage to optic nerve axons, retinal fibers and occipital cortex are also affected [26, 27]. Triggers for optic neuropathy development are increased intraocular pressure (IOP) or decreased cerebrospinal fluid (CSF) pressure in the retrobulbar portion of the optic nerve. However, even when stable IOP compensation is achieved medicamentally or surgically, visual function loss is still observed in the every fifth patient [8]. Of considerable role here are such risk factors as arterial hypotension, low perfusion pressure, a vasospastic syndrome, diabetes mellitus, and myopia [11, 21]. All this gives a reason to be less dismissive of a neuroprotective therapy with a purpose to stabilize the glaucomatous process and to create conditions for preserving the visual system (VS) function. Meanwhile, the difficulties which ophthalmologists face when making a decision on a neuroprotective therapy are well-known [28]. Taking into account a progressive character of the disease, it is required to influence the affected structures having preserved their morphological and functional normality. Neurodegeneration is caused by elevated IOP and reduced blood flow in the optic nerve [24, 30, 35]. As the optic nerve becomes more hypoxic, mitochondria get damaged, which leads to the formation of reactive oxygen species and cell stress [17], axonal transport disorders, neuroinflammation [25], glutamate-induced excitotoxicity [9], and autophagy [18, 37]. Low level laser radiation (LLLR) has an anti- edematous, anti-inflammatory, desensitizing, immunomodulating, and antioxidant action as well as stabilizes retinal lysosome membranes [1], stimulates neurogenesis and synaptogenesis, reducing apoptosis and cognitive impairment [12, 33], which substantiates the use of LLLR in the complex neuroprotective therapy in patients with glaucomatous optic neuropathy. Since glaucoma development is partially mediated by the increased number of reactive oxygen species, antioxidant methods have been developed for treatment of the disease. Experimental studies have shown that antioxidants, including coenzyme Q10, alpha-lipoic acid, superoxide dismutase, and ginkgo biloba, decrease ganglion cell loss [6, 19, 34], which potentially determines their efficacy in treating glaucoma [15]. Besides, it is common for glaucoma patients to be interested in the disease required food ration and in possibility of their dietary habits to contribute to the improvement of their state. In 2012, Wan MJ from Toronto University analyzed the questionnaires obtained from glaucoma patients and noticed that the patients received herbal preparations, dietary modifications, and vitamin/mineral supplements in 34.5%, 22.7%, and 18.8% of cases, respectively; and 40.5% of the examinees believed that the treatments were helping their glaucoma [36]. So, the further study on the efficacy of a long-term vitamin and antioxidant complex in glaucoma patients is of a significant interest [10, 31]. The purpose of the present paper was to study the efficacy of complex neuroprotection in patients with glaucomatous optic neuropathy (GON) using sequential laser radiation of the papillomacular bundle followed by a six-month vitamin and antioxidant supplement therapy. Material and methods We followed up 27 patients (43 eyes) with advanced open angle glaucoma, verified GON, and IOP stabilized surgically (21 patients) and/or medicamentally. The patients’ age averaged 63.8 (SD; 6.8), ranging from 54 to 78; 58% of the patients were of the working age (54 to 62 y/o); a men/women ratio was 17/10 (53%/47%). A treatment course included 10 every-day sessions of laser stimulation (LS) using a SM-4.3 unit (wavelength, 650 nm; irradiance, 0.4 mW/cm2; treatment timing, 240 s on the papillomacular bundle) and additional directed laser radiation of the optic disc (OD) using a 90 D lens (treatment timing, 60 s). Thereafter, patients received a vitamin supplement with 1 mg resveratrol: one capsule daily for 6 months. Patients underwent comprehensive clinical and functional ophthalmological examination, including visual acuity assessment, standard automated perimetry, ophthalmoscopy, fluorescence angiography of the visual system (VS), phosphene threshold current (PTC), and ophthalmic rheography (ORG), which was performed at baseline, immediately after a course of LS, and after 3 and 6 months of a vitamin and antioxidant supplementation. Perimetry was performed using Humphrey 750-і Visual Field Analyzer ("Carl Zeiss", the USA) and SITA Central 30-2 test, designed to study the central visual field within 30º. Findings were assessed using total scores of threshold values of the retinal light sensitivity (dB) in each visual field point studied; Mean Deviation (MD) of light sensitivity of the retina from the norm; and overall Рattern Standart Deviation (PSD). In addition, we analyzed Visual Field Index (VFI) which indicates a percent of normal visual field points studied. The calculations were made using Statistica 10 software package (StatSoftInc.). IOP changes were assessed using the Friedman rank analysis of variance with the consequent Wilcoxon signed-rank test. To demonstrate the changes in the differences, the data obtained are given as the median (Ме) and the standard deviation (SD). Spearman rank correlation coefficient was also calculated. Results The mean baseline best-corrected visual acuity (BCVA) value was 0.65 (SD; 0.29). The mean BCVA improved by 31% (р=0.0001) immediately after a course of laser therapy and decreased after 3 months of a supplementation with resveratrol (1m); however, after 6 month of a vitamin and antioxidant supplement therapy, the mean BCVA was equal to 0.75 (SD; 0.3), which was significantly higher, by 0.17 (26%), than that at baseline (χF2 = 74.5; p=0.000) (Table 1).
The baseline IOP value was compensated and equaled 16.3 (SD; 2.34) mm Hg. No significant changes in IOP were revealed immediately after a course of laser therapy, and after 3 and 6 months of a supplementation with resveratrol (1m). The mean PTC value equaled 84.9 (SD; 18.30) μA at baseline and decreased to 76.1 (SD; 12.1) μA by 10% (Р<0.05) after a LS course. The PCT value tended to decrease after 3 months of a supplementation and, at 6 months, visual system sensitivity was 76 μA, which was significantly lower, by 8.9 μA (10%), than that at baseline (χF2 = 52.9; p=0.000) (Table. 2).
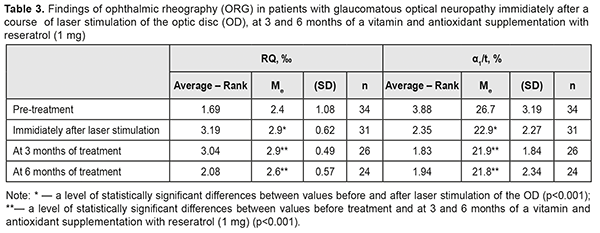
RQ blood circulation in the eye was decreased to 2.4 (SD; 1.08) ‰ at baseline; a LS course of the optic nerve and papillomacular bundle improved blood circulation in the eye to 2.9 (SD; 0.62) %, i.e. by 21%. Although the RQ value tended to decrease after 3 months, it remained significantly higher, by 8%, after 6 months of a supplementation therapy as compared to the baseline one (χF2 = 24.2; p=0.0002) (Table 3).
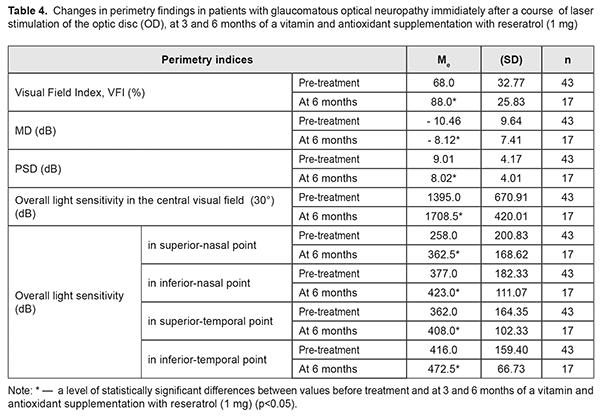
At baseline, the patients with GON were α1/t recorded to have vasospasm with the mean α1/t value of 26.7 (SD; 3.19)% (Table 3). The mean α1/t value decreased by 14% after a LS course. Studies after 3 and 6 months of a supplementation course showed a significant decrease in the mean α1/t value to 21.8 (SD; 2.34) %, i.e. by 18%, which indicated a decreased tension of intraocular vessels (χF2=47.13; p=0.0001). Over the follow-up period, the most informative were the perimetry values obtained during six months of a complex neuroprotective therapy. So, in most patients with GON, the overall value of the retinal light sensitivity in central vision field was increased; MD of overall light sensitivity of the retina from the norm was decreased; РSD was decreased; and VFI was increased (Table 4).
Thus, the total score of light sensitivity in the central visual field increased by 23 %: from 1395.0 (SD; 670.91) dB to 1709.00 (SD; 420.01) dB (р<0.001), before and after treatment, respectively. Analyzing the data, it can be said that the improvement is achieved due to increased light sensitivity in all visual field points studied. The maximal (by 41%) and minimal (by 12%) increase of Ме was, in the inferior-nasal and superior-temporal VF points, respectively. A significant decrease (by 22%) was also noted in MD of overall light sensitivity from the norm, from baseline -10.46 (SD 9.64) dB to post-treatment -8.12 (SD 7.41) dB, (р<0.001); PSD was also decreased, by 11%, from baseline 9.01 (SD 4.17) dB to 8.02 (SD 4.01) dB (р<0.02), which indicates reduction in the depth and prevalence of local visual field defects. Based on VFI values, if light sensitivity was within the norm in 68% of the VF points studied at baseline, then, after a six-month neuroprotective therapy, it increased by 23% and was equaled 88% (р<0.001). Successful outcome which was obtained after the treatment course was stable over the period of six months. Analyzing the data obtained after a neuroprotective LS course with a subsequent vitamin antioxidant supplementation therapy with reseratrol (1 mg) revealed an apparent correlation between an increase in overall light sensitivity of the retina and an increase in the blood circulation in the eye (rs=0.71, р<0.01) as well as a decrease in threshold current of visual system sensitivity (rs=-0.84, р<0.01). Discussion One of the key mechanisms in development and progression of glaucoma is low ocular blood flow caused by ischemia and ischemia-accompanying hemodynamic and microcirculatory changes [38]. Our combined therapy increased retinal blood flow immediately after LS and maintained a LS action throughout 6 months of a supplementation with resveratrol (1 mg). Our studies are in agreement with the experimental findings of V.V. Neroev et al. (2016) and T.N. Kiseleva (2016) which have given evidence of increased blood flow in retrobulbar arteries in the post-ischemic period against the background of a long-term supplementation of antioxidant resveratrol, which is a component of a vitamin antioxidant supplement Nutrof®Total [3, 4]. And the findings of Engler M.B. (1992) have shown the effect of omega 3 fatty acids, including modulation of the release of intracellular calcium ion to stabilize blood circulation [10]. Our study demonstrated that the elevated tension of intraocular vessels decreased by 18% in glaucoma patients over a six-month vitamin antioxidant supplementation with resveratrol (1 mg), which is consistent with our previous results of a combined therapy for asthenopia students [2]. After a treatment course, a significant improvement was noted in standard automated perimetry findings: overall light sensitivity increased by 23%; Mean Deviation (MD) of light sensitivity of the retina from the norm decreased by 22%; overall Рattern Standart Deviation decreased by 11%; Visual Field Index increased to 88% in most patients with GON. Successful outcome which was obtained after the treatment course was stable over the period of six months. The positive result remained stable for six months. The functional improvement in patients with GON can indirectly indicate a neuroprotective effect of a proposed supplement therapy as well as an increased functional activity of the retinal neurons. Our findings are in agreement with those by Miyauchi O. (2001) which have confirmed that photoreceptors are better preserved and transient retinal ischemia-induced damage to the retina is reduced, which provides an antiapoptotic action after omega 3 fatty acids application [23]. The neuroprotective action might be supported by docosahexaenoic acid [16] which is a component of a vitamin supplement with resveratrol (1 mg). Experimental findings by Liang H.L. (2006) have shown that LLLR protects neurons from apoptotic cell death and our data, indicating the stabilization of the process in patients with glaucomatous optic neuropathy, explains a neuroprotective action of the method combining LS and a six-month vitamin antioxidant supplementation with resveratrol (1 mg). Our results are in agreement with the findings of other researchers indicating that a diet including antioxidants, polyunsaturated fatty acids, and nutraceuticals can have an effect on glaucoma progression [5; 7; 13-15; 29; 32] . The revealed stabilization of the visual system state is consistent with findings of the statistic perimetry and emphasizes the efficacy of a complex neuroprotective therapy. Conclusions A combined treatment including LS of the optic nerve and the papillomacular bundle followed by a six-month vitamin and antioxidant supplement therapy with resveratrol (1 mg) in patients with glaucomatous optical neuropathy contributes to: an increase in visual acuity from 0.65 to 0.82 (by 26%); an increase in the visual system sensitivity, evidenced by a decrease of phosphene threshold current from 84.9 μA to 76 μA (by 8.9 μA or 10%); an improvement in ocular blood flow from 2.4 to 2.6‰ (by 8%); a decrease of intraocular vasospasm from 26.7 to 21.8% (by 18%); and a significant decrease in overall retinal light sensitivity from 1395.0 to 1709.0 dB (by 23%). It also confirms: an increase in conduction of optic nerve fibers; an improvement of the papillomacular bundle function; and stabilization of the glaucomatous process during six months.
References
|