J.ophthalmol.(Ukraine).2018;1:19-25.
|
https://doi.org/10.31288/oftalmolzh201811925 Efficacy of laser stimulation of the retina with subsequent nutrient supplementation for treatment of asthenopia in students O.V. Guzun, Cand Sc (Med), N.I. Khramenko, Cand Sc (Med) SI “Filatov Institute of Eye Diseases and Tissue Therapy of NAMS of Ukraine"; Odessa (Ukraine) E-mail: olga.v.guzun@gmail.com TO CITE THIS ARTICLE: Guzun OV, Khramenko NI. Efficacy of laser stimulation of the retina with subsequent nutrient supplementation for treatment of asthenopia in students. J.ophthalmol.(Ukraine).2018;1:19-25. https://doi.org/10.31288/oftalmolzh201811925
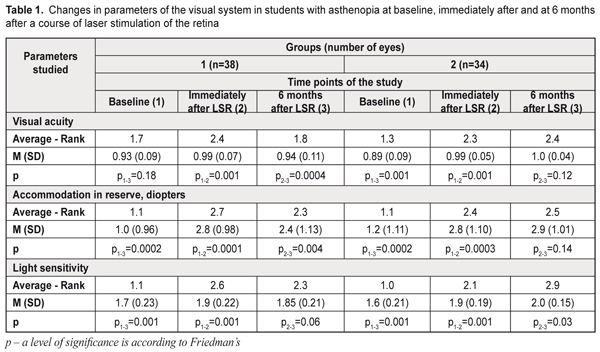
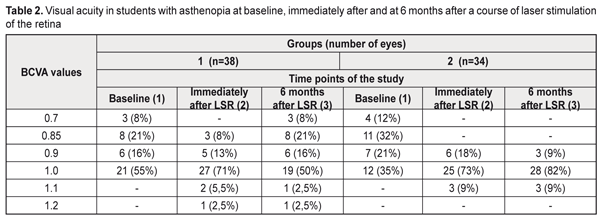
Background. Asthenopia is a key point in information fatigue syndrome. The incidence of asthenopia syndromes (one or several) among students is 57-89.9%. The recent data have evidenced that dietary supplements with a combination of omega-3 fatty acid, bilberry extract and lutein reduce asthenopia symptoms. Purpose. The purpose was to assess the efficacy of a course of laser stimulation of the retina with a subsequent nutrient supplementation in students with asthenopia. Material and Methods. We performed comprehensive examination of 36 students (72 eyes), aged 18 to 25, with asthenopia signs. There were two groups: group 1, 19 students (38 eyes); group 2, 17 students (34 eyes). All study eyes underwent 10 every-day sessions of diode laser stimulation of the retina (LSR) (wavelength, 650 nm; irradiance, 0.4 mW/sm²; treatment timing, 300 s). On completion of a course of LSR, the students of group 2 were recommended a Nutrof®Total vitaminous antioxidant complex, one capsule daily for 6 months. Results. A course of laser stimulation of the retina and a 6 month nutrient supplementation for asthenopia students made it possible to improve visual acuity by 12% in 91 % of the students, to recover the reserves of accommodation over 3 D in 73% of the students, to improve the vascular tone by 34% in 59% of the students and light sensitivity of retinal cones by 25% in 82% of the students; asthenopia graded from moderate to mild in 91% of the students; the overall emotional state was normalized in all students by 37% according to a HADS scale. Conclusions. A course of LSR and a 6 month nutrient supplementation enables to improve students’ overall health and professional activity while reduced asthenopia creates conditions for health improvement, better quality of studying and open career opportunities. Key-words: asthenopia in students, laser stimulation of the retina, nutrient supplementation Background Nowadays, computers, tablets, and smarthones are widely used, which has induced psychophysical problems in students worldwide. Students comprise a large group of intellectual workers and this work requires efforts of vision, memory, attention, and mental processes. This leads to the violation of rest and meals regimen, over-fatigue, performance impairment, and development of psychosomatic disease. So, the issues of students’ health maintenance and disease prevention are becoming more and more relevant today. Asthenopia is a key point in information fatigue syndrome. According to domestic and foreign literature, asthenopia occurs in 60-90% of display uses [1, 2]. The incidence of asthenopia syndromes (one or several) among students is 57-89.9% [3, 4, 5]. Besides, an overuse of smartphones can cause such problems as blurred vision while reading, double vision, eye dryness, and neck, wrist, or back pain [5, 6, 7]. In particular, fatigue related to prolonged work at a computer display is defined as computer vision syndrome (CVS) [8, 9, 10] with ocular symptoms and musculoskeletal symptoms (neck and shoulder pain). The main CVS symptom is asthenopia which, sometimes, is painful for a patient and affects the quality of life [11]. Smartphone constructions are being improved very quickly and high resolution displays can have certain advantages since they provide a brilliant image. However, D. J. Kim and colleagues (2017) have revealed in healthy people a significantly expressed visual fatigue and discomfort which are associated with viewing smart mobile devices with state-of-the-art display technology [12]. According to World Health Organization data (2016), 27 % of European population, which is almost a third, experience anxiety and depression conditions. It has been shown that 45% of smartphone users feel anxiety when they are not holding their smartphone [13]. A world literature review has shown that 11 to 33% of students have sleep disturbances, anxiety, and depression [14, 15]. Asthenopia (eye strain) is characterized by visual symptoms (blurred vision, double vision), ocular symptoms (burning, pain, redness, tearing, nictation, photophobia, and nictitating spasm), and concomitant psycho-emotional disorders (irritation, depression, anxiety, disturbance) [16]. Changes in the accommodative apparatus of the eye lead to impaired accommodative convergence and low fusion convergence [17]. This download trend is increased by blood flow impairment in the ciliary muscle, disorders in regulatory mechanisms of vegetative segmental formations, dystonia of cerebral vessels, mainly of a parasympathetic type, against the background of the low tonus of the sympathetic nervous system. O.A. Gromova et al. (2011) have shown that omega-3 polyunsaturated fatty acids (PSFA) play the leading role in the healthy growth and functioning of the brain and central nervous system [18]. Eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) are known to have an effect on the lipid spectrum, vascular tone, and blood clotting [19]. Omega-6 PSFA prevails in the food ration of students. Increased consumption of saturated and trans fats (margarine, fried food, French fries, chips etc.) results in disorders in fat metabolism in the body, hormone imbalance, and anxiodepressive symptoms. Normally, an omega-6/omega-3 ratio must be 4:1. However, the consumption of olive oil and fatty fish which are the main source of omega-3 PSFA (EPA and DHA) remains low. In such a dietary regimen, there is a shift towards an increase of omega-6 PSFA, and the omega-6/omega-3 ratio can be 20/1. The recent data have evidenced that dietary supplements with a combination of omega-3 fatty acid, bilberry extract, and lutein reduce asthenopia symptoms [20] and eight week consumption improves some objective and subjective symptoms of eye fatigue induced by visual loads [21]. J.M. Stringham et al. (2017) have determined that a long lasting carotinoid intake leads to a significant increase in optical density of the macular pigment, to a decrease of eye strain and fatigue, as well as to better sleep quality [22]. Our previous studies have shown that resveratrol normalizes a tone of intraocular vessels [23]. The purpose of the present paper was to assess the efficacy of a course of laser stimulation of the retina with a subsequent nutrient supplementation in students with asthenopia. Material and Methods We performed comprehensive examination of 36 students (72 eyes), aged 18 to 25, with asthenopia signs. There were two groups: group 1, 19 students (38 eyes); group 2, 17 students (34 eyes). All study eyes underwent 10 every-day sessions of diode laser stimulation of the retina (LSR) (wavelength, 650 nm; irradiance, 0.4 mW/sm²; treatment timing, 300 s) using a diode laser SM-4.3 unit. On LSR completion, the students of group 2 were recommended a Nutrof©®Total vitaminous antioxidant complex, one capsule daily for 6 months. The capsule contains omega-3-essential fatty acids (EPA, 40%; DHA, 20%); Vitamin C, 60mg; Vitamin E, 10mg; Tagetes Erecta extract, 65mg (Lutein, 10mg; Zeaxanthin, 2mg); 83% Zinc, 10mg; Vitis Vinifera extract, 5mg (Resveratrol, 1.0mg; Copper, 500ug; Selenium, 25ug; glutathione). All students were recommended a healthy lifestyle (controlled visual load), eye gymnastics, balanced diet, smoking and alcohol drinking cessation, physical activity, and good quality sleep. Functional ophthalmological examination included visual acuity test, refractometry, ultrasound diagnostics, pachymetry, biomicroscopy, measurements of light sensitivity of the photoptic afferent system, the assessment of reserve of accommodation by A.I. Dashevskii, and rheoophthalmography assessment of volume of blood circulation (RQ, ‰) and a tone of intraocular vessels (α1/T, %). To assess asthenopic complaints we calculated a coefficient of visual asthenopia syndrome (CVAS, scores) [24]. When filling in a questionnaire, a patient was asked to assess a degree of pronouncement for vision complaints using a 10 score scale where: 0 scores: no complaints; 10 scores: a maximally pronounced subjective symptom. CVAS was calculated as total score of all objective symptoms of asthenopia: 0 scores: no asthenopia symptoms; 10 to 30 scores: mild asthenopia; 40 to 60 scores: moderate asthenopia; 70 to100 scores: high asthenopia. To reveal and to assess depression and anxiety we used the hospital anxiety and depression scale (HADS) designed by A. Zigmond and colleagues (1983) for general medical practice [25]. When interpreting the data we considered a total score of anxiety and depression values where: 0 to 7 scores: normal, no anxiety and depression; 8-10 scores: subclinical anxiety/depression; 11 and more: clinical anxiety/depression. It took 5-10 minutes to complete the questionnaire. Statistical assessment of changes in values was performed using Friedman’s ANOVA test followed by the Wilcoxon signed-rank test to assess the paired difference. Changes in differences are presented using mean (M) and standard deviation (SD). To assess the relationship between study variables we used Spearman correlation coefficient (rs). Calculations were performed using STATISTICA 10.0 software (StatSoft Inc.). Values р<0.05 were considered statistically significant. Results and Discussion The baseline best uncorrected visual acuity (BCVA) in asthenopia students was 0.93 (SD, 0.09) and (SD, 0.09) in group 1 and group 2, respectively (Table 1). The frequency analysis showed that, BCVA in group 1 was ≥1.0 in 55% of the eyes at baseline, in 79 % of the eyes immediately after a course of LSR, and in 55% of the eyes at 6 months after a course of LSR. In group 2, BCVA was ≥1.0 in 35% of the eyes at baseline, in 82 % of the eyes immediately after a course of LSR, and in 91% of the eyes after a 6 months course of nutrient supplementation (Table 2).
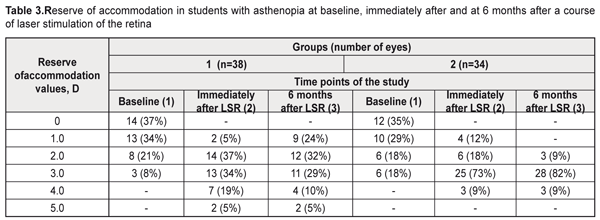
Table 1 demonstrates that at baseline and at 6 months of nutrient supplements there was a statistically significant increase (χF2=46.3; p=0.00001) in the mean BCVA values in the group 2 patients: after a 6 months nutrient supplementation this value increased by an average 12% in 31 eyes (91%). In group 1, no significant changes were noted within this follow-up period. No significant differences between groups in all indices of the functional state of the visual system before and immediately after a course of LSR were noted. Accommodation measurements showed the absence of reserve of accommodation in 14 eyes (37%) in group 1 and in 12 eyes (35%) in group 2 (Table 3). After a LSR course, the accommodation reserve values increased in all students: to 2.8 (SD, 0.98) D, i.e. by 1.8 D, in group 1 and to 2.8 (SD, 1.10) D, i.e. by 1.6 D, in group 2 (Table 1).
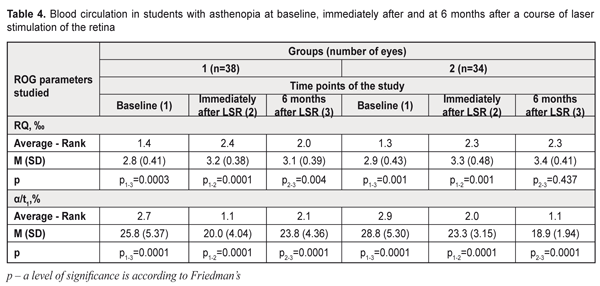
At 6 months after a course of LSR, the reserve accommodation values decreased to 2.4 (SD, 1.13) D, i.e. by 14%, in group 1 while, in group 2, an insignificant increase to 2.9 (SD, 1.01) D was noted as compared with the post-LSR values of resrve of accommodation. After 6 months of nutrient supplementation, the mean reserve accommodation values statistically significantly increased in the group 2 students (χF2=54.5; p=0.0001): this index was > 3D in 73% vs. 54% of the group 1 students. Light sensitivity of the macula (LSM) which reflexes compensatory and adaptive mechanisms of the afferent system also improved after a course of LSR in both groups: by 12% and 18% in group 1 and 2, respectively (р<0.001). 6 months after a course of LSR, LSM 7 minutes into the study increased to 2.0 (SD, 0.15) (р<0.001), i.e. by 25%, in 82% of the group 2 students; while, in group 1, this index decreased to 1.85 (SD, 0.21) and was within the normal ranges only in 47% of the students. As it can be seen in Table 1, at 6 months of the supplementation with a vitaminous antioxidant complex, the students of group 2 had a statistically significant increase of light sensitivity of the macula (χF2=62.5; p=0.00001), which was confirmed by the increased mean LSM values, at average by 25%: after a 6-month nutrient supplementation, the LSM values reached the normal ranges in 28 eyes (82%). In group 1, by 6 months, the LSM values reached the normal ranges in 18 eyes (47%), which is 1.7 times less than in group 2. RQ blood circulation in the eye after a course of LSR improved, at average, by 14% in both groups: by 3.2 (SD; 0.38) ‰ and 3.3 (SD; 4.48) ‰, in group 1 and 2, respectively (Table 4). At 6 months, no significant changes in blood circulation were noted in the groups. Based on the literature data, the improved working capacity of the ciliary muscle is directly related to improved hemodynamics in the ocular vessels. Insufficient blood supply is one of the main mechanisms leading to impaired accommodation. Studies by T.N. Kisileva and colleagues (2013) have shown a significant increase of rheography coefficient by 66% and a decrease of asthenopia symptoms after a course of transscleral laser stimulation of the ciliary body [26]. At baseline, vascular spasm was revealed in 95% of the group 1 students and in 97% of the group 2 students. After a course of LSR we noted a significant improvement of the tone of intraocular vessels (α/t1) by 22% and 19%, i.e. to 20.0 (SD; 4.04) % and 23.3 (SD; 3.15) %, in the students of group 1 and 2, respectively (p<0.001) (Table 4). Studies at 6 months after LSR revealed a further decrease in the tone of the intraocular vessels to 18.9 (SD; 1.94) %, i.e. by 19%, in 59% of the group 2 students (p<0.05), while in the group 1, the spasm of the intraocular vessels increased to 23.8 (SD; 4.36) % (p>0.001), i.e. by 19% (p>0.001). Hence, during the whole follow-up period, the intraocular vascular spasm decreased by 34% in the students of group 2 (p<0.001). As it can be seen in Table 4, 6 months of a nutrient supplementation resulted in a significant increase in the mean values of the tone of the intraocular vessels (χF2=62.7; p=0.00001): the normal ranges of the intraocular vascular tone were noted in 20 eyes (59%) in group 2. At 6 months, the vascular spasm was noted in 86 % of the group 1 students.
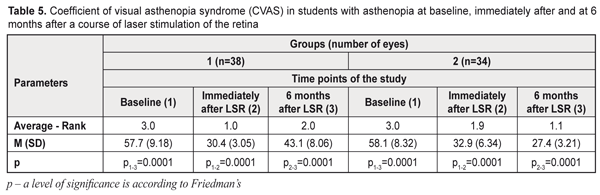
CVAS study results The baseline CVAS corresponded to high asthenopia in 45 % and 35% in the students of group 1 and 2, respectively, and to moderate asthenopia in 55% and 65% in the students of group 1 and 2, respectively. The post-LSR CVAS decreased to mild asthenopia in 53% and 38% of the students, respectively, in group 1 and 2; moderate asthenopia was noted in 47% and 62 % of the students and averaged 30.4 (SD; 3.05) and 32.9 (SD; 6.34) scores, in group 1 and 2, respectively (Table 5). A 6 month nutrient supplementation in group 2 resulted in a shift from moderate to mild asthenopia, to 27.4 (SD; 3.21) scores (p<0.001), in 91% of the students; while in group 1 the CVAS values increased to 43.1 (SD; 8.06) scores, (p<0.001), i.e. by 25%, with the impaired subjective state according to asthenopia pronouncement.
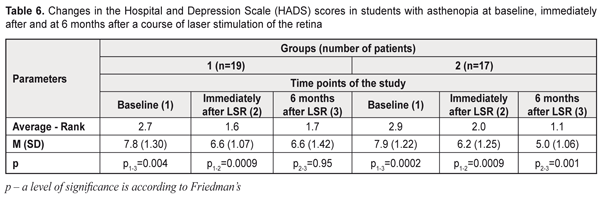
HADS questionnaire study results At baseline (before treatment), 12 students (63%) in group 1 and 10 students (59%) in group 2 had subclinical anxiety and/or depression (with HADS scores ≥ 8). Four students (21%) in group 1 and three students (18%) in group 2 had clinical anxiety and/or depression after a course of LSR, 6.6 (SD; 1.07) and 6.2 (SD; 1.25) scores, respectively, (Table 6). At 6 months after a course of LSR, four students (21%) in group 1 and 17 students (100%) in group 2 had normal HADS scores. At 6 months, the overall emotional distress score improved by 15% in 8 students (42%) in group 1 and by 37% in 17 students (100%) in group 2.
The students after a 6-month supplementation with a vitaminous antioxidant complex were revealed moderate correlation relationships between: a decrease of an asthenopia degree and an increase of reserve of accommodation (rs=-0.44, р<0.01) as well as a decrease of the CVAS (rs=0.5, р<0.01); between a decrease of the tone of intraocular vessels and a decrease in anxiety/depression score values (rs=0.56, р<0.05), a decrease of the CVAS (rs=0.42, р<0.05), an increase in light sensitivity of the macula (rs=-0.34, р<0.05), and a decrease of the asthenopia degree (rs=0.36, р<0.05). Our findings are consistent with literature data which have revealed a moderate correlation relationship between a good sleep, psychological state and consumption of green leafy vegetables in computer users with asthenopia [27]. It is important to emphasize that the 6 month follow-up revealed a significant decrease in anxiety and depression in the students after a nutrient supplementation, which is consistent with findings of other studies on a positive effect of omega-3 fatty acids on depression and anxiety symptoms [28]. Our findings showed a stabilization of visual function in these patients, which is supported by other authors who have marked an increase in macular pigment optical density during 48 weeks with a further improvement of visual function [29] as well as decreased asthenopia [30] after a nutrient supplementation. A nutrient supplementation course as additional protection of eye tissues in students with asthenopia after a LSR course resulted in the normalization of the tone of intraocular vessels, the increased activity of compensatory and adaptive mechanisms which control light sensitivity of the photoptic afferent system as well as in decreased asthenopia and normalized overall emotional state of the students. Conclusions A course of laser stimulation of the retina and a 6 month Nutrof® Total supplementation for asthenopia students enabled to improve visual acuity by 12% in 91 % of the students, to recover reserves of accommodation over 3 D in 73% of the students, to improve the vascular tone by 34% in 59% of the students and light sensitivity of retinal cones by 25% in 82% of the students; asthenopia graded from moderate to mild in 91% of the students; the overall emotional state was normalized in all students by 37% according to a HADS scale.
A 6 month nutrient supplementation enables to improve students’ overall health and professional activity while decreased asthenopia creates conditions for health improvement, increased quality of studying and open career opportunities. References
|