J.ophthalmol.(Ukraine).2018;2:45-49.
|
https://doi.org/10.31288/oftalmolzh/2018/2/4549 Stability of lysosomal membranes of ocular neuronal structures in the rabbit model of glaucoma in the presence of experimental diabetes mellitus V. R. Yurevych, Cand Sc (Med), Ass Prof Danylo Halytsky Lviv National Medical University; Lviv (Ukraine) E-mail: yurevich@yahoo.com TO CITE THIS ARTICLE: Yurevych VR. Stability of lysosomal membranes of ocular neuronal structures in the rabbit model of glaucoma in the presence of experimental diabetes mellitus. J.ophthalmol.(Ukraine).2018;2:45-59. https://doi.org/10.31288/oftalmolzh/2018/2/4549
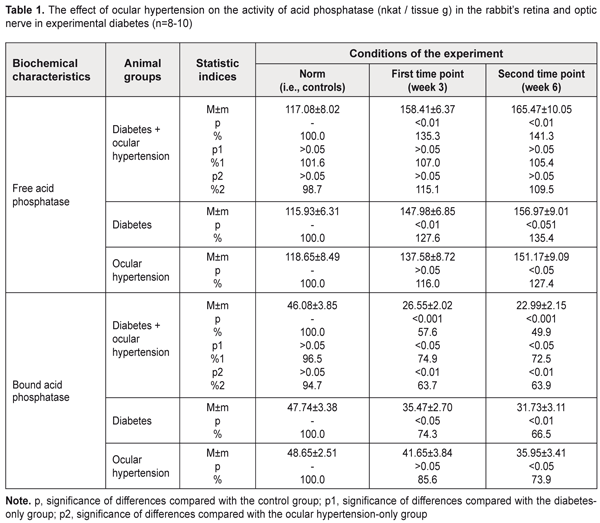
Background: Pathogenetic mechanisms of glaucoma remain largely poorly understood. Some authors consider diabetes as a major risk factor for glaucomatous damage. Determination of the state of lysosomal membranes of the retina and optic nerve in primary glaucoma associated with diabetes is important for establishing the pathogenesis of this combined disorder. Purpose: To investigate the structural and functional stability of lysosomes in a rabbit model of experimental glaucoma (ocular hypertension) associated with streptozotocin-induced diabetes. Materials and Methods: A total of 34 adult rabbits were used in the study to model streptozotocin-induced diabetes only, glaucoma (ocular hypertension) only or glaucoma associated with diabetes. The activity of the marker lysosomal enzyme, acid phosphatase, was determined in the retinal and optic nerve tissue. Results: Stability of lysosomal membranes in the neuronal ocular tissue was found to be sharply decreased in the model of glaucoma associated with diabetes. The activity of the marker lysosomal enzyme, acid phosphatase, changed more markedly in the model of glaucoma associated with streptozotocin-induced diabetes than in those of diabetes only or glaucoma only. Conclusion: Lysosomal dysfunction is a pathogenetic phase of the glaucomatous process in the presence of diabetes mellitus. Keywords: glaucoma, ocular nerve tissue, lysosomal dysfunction, rabbits Introduction Treatment of primary glaucoma remains a major challenge for ophthalmologists around the world. In addition, the disease is a phenomenon of both medical and social importance, being a leading cause of irreversible blindness, visual impairment and visual disability [1-3]. Although multiple tools are available in the pharmacological, technical and surgical armamentaria of the glaucomatologist, they are often of low efficacy. This is due to the facts that (1) pathogenetic mechanisms of the disease are complex and insufficiently investigated and (2) treatment and prevention approaches are usually symptomatic rather than pathogenetic. The incidence of glaucoma has been found to be significantly higher in patients with diabetes mellitus compared with non-diabetics. The results of some meta-analyses suggest that diabetic patients are at significantly increased risk of developing primary open-angle glaucoma [4-5]. Given a high prevalence of diabetes mellitus among patients with glaucoma, and because some phases involved in the pathogenesis of primary glaucoma are similar to those of diabetes mellitus, pathogenetic treatments for these disorders are of special importance. Probably it is the pathophysiological and metabolic changes in glaucoma (especially, in the presence of diabetes mellitus) that primarily due to the production of free radicals lead to major retinal and optic nerve damage. In glaucoma or diabetic retinopathy, if free radical oxidation is activated, active oxygen forms and their metabolites exert cytotoxic effect on the retina and optic nerve. Free radicals cause oxidation of biological molecules, which, in turn, triggers a series of lipid peroxidation reactions in membranes, and induces damage to nucleic acids and proteins. The loss of stability in the lysosomal membranes of the retina and optic nerve has been demonstrated in the rabbit model of glaucoma [6]. These changes evolved as the glaucomatous process progressed. Solubilization of the marker lysosomal enzyme, acid phosphatase, increases with structural damage to intracellular membranes, and the authors [6] found that its activity significantly decreased as early as the initial phase of the process. At the end of the follow-up, the activity of a sedimentable form of the enzyme in the retina and optic nerve was found to be reduced by one third compared to the norm. The loss of stability in the lysosomal membranes of the retinal and optic nerve cells which was found in the experimental model of glaucoma could be regarded as the consequence of oxidative stress developing in this condition. The role of free radical oxidative processes in diabetic retinopathy is already well known [7-8]. An abrupt increase in free-radical lipid oxidation due to loss of balance between the free radical production and the capacity of antioxidant systems in the retina plays an important role in the mechanism of ultrastructural degeneration of the RPE in diabetes mellitus. Labilization of the lysosomal membranes of the retinal pigment epithelium has been demonstrated in experimental streptozotocin-induced diabetes [9]. Therefore, our attention has been drawn to the issue of the state of the lysosomal membranes of the retinal and optic nerve cells in primary open-angle glaucoma (POAG) associated with diabetes mellitus. The purpose of the study was to investigate the structural and functional stability of lysosomes in a rabbit model of experimental glaucoma (ocular hypertension) associated with streptozotocin-induced diabetes. Materials and Methods A total of 34 adult Chinchilla rabbits with a weight of approximately 3 kg were used in the study. All procedures performed in the study were in accordance with the ethical standards of the Council of Europe's Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, and Guidelines on the Conduct of Experimental Animal Studies, as approved by the Order of the Ministry of Health of Ukraine and the Law of Ukraine on the Protection of Animals from Ill Treatment No. 1759-VI dated 15 December 2009. The rabbits were divided into four groups: Group 1 (a control group of intact animals, n=8), Group 2 (experimental glaucoma (ocular hypertension) associated with streptozotocin-induced diabetes, n=10), Group 3 (experimental group with diabetes only, n=8), and Group 4 (experimental group with glaucoma only, n=8). Each group was subdivided into two subgroups, depending on the study time points used (subgroup 1, three weeks; subgroup 2, six weeks). Diabetes was induced with a single intravenous injection of streptozotocin, 65 mg/kg body wt [10]. Seven days after inducing diabetes, ocular hypertension was induced in the presence of a stable blood glucose level. To induce ocular hypertension, 0.2% methylcellulose was administered into the anterior chamber [11]. When inducing ocular hypertension, animals were anesthetized generally with ketamine (50 mg/kg), and locally with 0.5% procaine hydrochloride instilled into the conjunctival sac 1 minute before injection. Intraocular pressure (IOP) was measured at baseline and at time points with a non-contact tonometer Topcon CT–80 (Topcon Corp., Tokyo, Japan) under local anesthesia. At week 3 or week 6 after inducing ocular hypertension, rabbits were euthanized by 100 mg/kg pentobarbital sodium injection administered intravenously via the marginal ear vein. The retina and optic nerve were immediately removed and put in fresh medium for subsequent removal of lysosomes. The tissues were suspended in the buffer containing 20 mM Hepes-KOH pH 7.5, 1.5 mM MgCl2, 0.5 mM EGTA, and 250 mg sucrose with polyvinyl pyrrolidone. A glass/Teflon homogenizer was used to prepare a tissue homogenate at 1:10 ratio with homogenization medium. Homogenates were centrifuged at 750 g for 10 min at 4°C for removal of nuclei and cell debris. Sediment rich in lysosomes was produced by further centrifugation of the supernatant at 6,000 g for 15 min at 4 °C. The resultant pellet was resuspended and subsequently used to determine the lysosomal enzymatic activity of the bound acid phosphatase. The activity of cytoplasmic isoform of acid phosphatase in the supernatant was measured. The method for determination of acid phosphatase activity is based on the determination of the level of paranitrophenyl phosphate (a free organic component of the substrate) that is produced from the action of the enzyme [12, 13]. To determine the activity of acid phosphatase, 0.1 mL of the extract of tissue under investigation was sequentially mixed in test tubes with 0.1 mL of substrate buffer solution (0.127% p-nitrophenyl phosphate solution in acetate buffer, pH 5.0). The test tubes with reaction solution were incubated at 37°C for 30 min, and the reaction was stopped by the addition of 1.0 ml NaOH solution cooled to 0°C. After incubation, optical densities were read on a spectrophotometer (Spekol 210, Carl Zeiss Jena, Germany) at a wavelength of 410 nm. The activity of acid phosphatase was calculated using a molar extinction coefficient obtained from a calibration curve of known extinction coefficients, and expressed in nkat per gram of tissue. The coefficient of variation was 7.8%. Statistical analyses were performed with SPSS 11.0 software. Statistical significance of difference was determined using Student's t-test. Results and Discussion We assessed the functional stability of lysosomal membranes of the ocular nerve tissue (retinal plus optic nerve cells) through the activity of the two forms of the acid phosphatase (AP) enzyme, free AP and bound AP. To assess the enzymatic activity, a lysosomal fraction of a homogenate of the tissue was obtained and underwent reaction. This data represented the activity of bound AP. The enzymatic activity was determined in the supernatant that contained no lysosomes and that represented the activity of free AP. Table 1 presents the data on the effect of glaucoma (ocular hypertension) on the activity of acid phosphatase in the retina and optic nerve in experimental diabetes in rabbits.
In Group 2 (diabetes plus glaucoma), the activity of free AP increased to 135% (i.e., 158.41±6.37) nkat/g, p < 0.01) and to 141.3% (i.e., 165.47±10.05) nkat/g, p < 0.01), as compared to a norm of 117.08±8.02 nkat/g, by weeks 3 and 6, respectively. In Group 3 (diabetes only), the activity of free AP increased to 127.6% (i.e., 147.98±6.85) nkat/g, p < 0.01) and to 135.4% (i.e., 156.97±6.31) nkat/g, p < 0.01), as compared to a norm of 115.93±6.31 nkat/g, by weeks 3 and 6, respectively. In Group 4 (ocular hypertension only), the activity of free AP increased to 116.0% (i.e., 137.58±8.72) nkat/g, p < 0.05) and to 127.4% (i.e., 151.17±9.09) nkat/g, p < 0.05), as compared to a norm of 118.65±8.49 nkat/g, by weeks 3 and 6, respectively. There were no statistically significant differences between the data sets from different experimental groups. However, it should be noted, that in Group 2 (diabetes plus glaucoma), the activity of free AP tended to be higher than in Group 4 (ocular hypertension only) at both time points. In Group 2 (diabetes plus glaucoma), the activity of bound AP decreased to 57.6% (i.e., 26.55±2.02 nkat/g, p < 0.001) and to 49.9% (i.e., 22.99±2.15 nkat/g, p < 0.001), as compared to a norm of 46.8±3.85 nkat/g, by weeks 3 and 6, respectively. In Group 3 (diabetes only), the activity of bound AP decreased to 74.3% (i.e., 35.47±2.70 nkat/g, p < 0.05) and to 66.5% (i.e., 31.73±3.11 nkat/g, p < 0.01), as compared to a norm of 47.74±3.38 nkat/g, by weeks 3 and 6, respectively. In animals with diabetes plus glaucoma, decreases in the activity of bound AP at time points 1 and 2 were 25.1% and 27.5% (p < 0.05), respectively, greater than in animals with diabetes only. In Group 4 (ocular hypertension only), the activity of bound AP decreased to 85.6% (i.e., 41.65±3.84) nkat/g, p < 0.05) and to 73.9% (i.e., 35.95±3.41) nkat/g, p < 0.05), as compared to a norm of 48.65±2.51 nkat/g, by weeks 3 and 6, respectively. Therefore, in all groups of animals, the acid phosphatase enzyme activity in the lysosomal fraction in the neuronal ocular tissue (i.e., inside lysosomes of retinal and optic nerve cells) decreased over time. This was evidenced by changes in the activity of bound AP. The situation was reversed for the activity of acid phosphatase outside the lysosomal fraction of the neuronal ocular tissue, with the activity of free AP in all experimental groups (with diabetes and/or glaucoma) significantly increased over time. The most substantial changes with regard to activities of these forms of the enzymes were observed in experimental diabetes plus ocular hypertension. Lysosomes are cell organelles that due to their function have a specific and rather stable membrane. Acid phosphatase is one of numerous hydrolases, specific lysosomal enzymes. As the presence of this enzyme outside lysosomes evidences of a loss of stability of the lysosomal membrane, acid phosphatase is a recognized marker of lysosomal membrane labilisation. Because the membrane is rather strong, only certain agents (like cytotoxic free radicals and lipid peroxidation products) are capable of destroying it. In this connection, we believe that, in glaucoma and/or diabetes mellitus, it is the oxidative stress that induces damage to lysosomal membranes, which was evidenced by the findings of imbalance between lysosomal and cytoplasmic (i.e., bound and free) forms of acid phosphatase in this experimental study. A cell culture study [14] demonstrated that chronic oxidative stress of trabecular meshwork cells resulted in decreased lysosomal activity and those authors suggested that such changes contribute to the progression of glaucoma. In addition, our previous rabbit study [15] demonstrated that, in increased IOP, hyperglycemia (1) exerted a membrane trophic effect on the ultrastructure of the anterior chamber angle and (2) sharply decreased the stability of lysosomal membranes in these tissues. Our present study was conducted under conditions similar to those used for the previous study, and demonstrated the presence of the same mechanism for destabilization of lysosomal membranes also in the ocular nerve tissue. Conclusion A sharp decrease in the stability of lysosomal membranes in the ocular nerve tissue was observed in the model of glaucoma (ocular hypertension) in the presence of experimental diabetes mellitus. In the rabbit model of ocular hypertension in the presence of experimental streptozotocin-induced diabetes, the activity of the marker lysosomal enzyme, acid phosphatase, in the optic nerve and retina, underwent more substantial changes than in the model of ocular hypertension only or diabetes only. We believe that lysosomal dysfunction is a pathogenetic phase of the glaucomatous process in the presence of diabetes mellitus. References
|