J.ophthalmol.(Ukraine).2017;3:48-55.
|
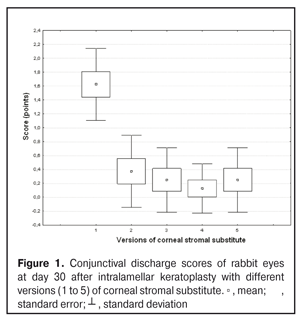
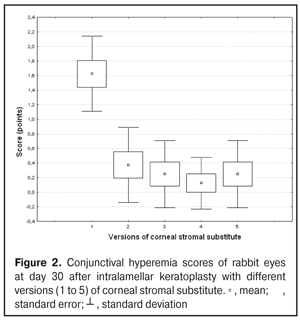
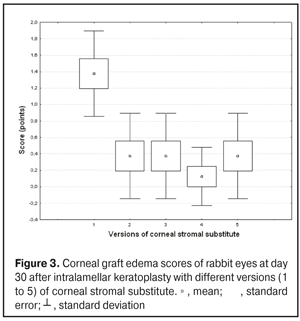
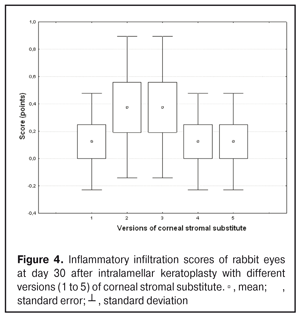
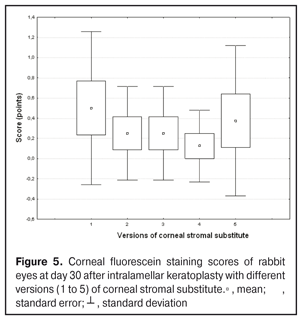
https://doi.org/10.31288/oftalmolzh201734855 Experimental study of efficacy of intralamellar keratoplasty with corneal stromal substitute developed from decellularized porcine cornea N.V. Pasyechnikova, Dr. Sc. (Med), Prof. B.M. Cogan, Cand Sc (Med) S.G. Kolomiichuk, Research Fellow Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine, Odessa, Ukraine E-mail: koganb@ukr.net Backrgound: Enhancing methods for decellularization of animal corneas for the development of bioengineered corneal models with the following keratoplasty makes it possible to overcome the shortage of human donor material. Purpose: To investigate the clinical efficacy of intralamellar keratoplasty (IKP) with corneal stromal substitutes (CSSs) developed using different decellularization methods in the animal study. Materials and Methods: Corneas were excised from enucleated pig eyes, and, to develop acellular CSSs, five methods of decelluarization of porcine corneas were used that were different in the time and conditions of incubation with detergents (0.5% sodium dodecyl sulphate or TRITON X-100) and proteolytic enzymes (like 0.1% papain). In addition, an ultrasonic apparatus of type СD 3800 А was used to further improve the removal of cells and acellular corneal debris. A total of 40 Chinchilla rabbits were used in the animal study. They were divided into 5 groups with different CSS versions. In each rabbit, the monocular IKP was carried out with one of the five CSS versions. Postoperatively, antimicrobial and anti-inflammatory therapy was instituted. Animals were examined every other day for 30 days postoperative. This included corneal fluorescein staining. In addition, the following was assessed ophthalmoscopically: conjunctival discharge, conjunctival hyperemia, state of the CSS, state of the host cornea, and, at late follow-up examinations, graft acceptance and whether graft-versus-host disease was present. Results: In rabbit eyes that underwent IKP with a version 4 CSS, the clinical scores for conjunctival discharge, corneal graft edema, inflammatory infiltration, corneal graft opacity and corneal fluorescein staining (0.13 points) were statistically significantly lower than in eyes that underwent IKP with other CSS versions. Location of inflammation in the cornea was found to be paracentral in eyes that improved clinically after IKP with version 1, 2, 3 or 5 CSS, and tended to be central (0.38 points) in those that underwent IKP with a version 4 CSS. Conclusion: The least apparent reaction was noted in the rabbit eyes that underwent IKP with a version 4 CSS, which makes this CSS promising for further investigations in pre-clinical and clinical studies. Key words: intralamellar keratoplasty, porcine cornea, corneal stromal substitute, experiment Introduction Today, in spite of advances in our knowledge of the etiopathogenesis of different anterior eye disorders, corneal disease is a major cause of loss of vision. For many cases with a severe clinical form of corneal injury, surgical treatment, namely, keratoplasty, is therefore the only effective option [1-3]. Because the application of such a radical treatment (as keratoplasty) is hampered by the risk of graft rejection in antigen incompatibility between graft and host, and by low availability of adequate donor material, developing alternatives to human donor cornea is of great importance [1, 4-13]. In the last decade, xenotransplantation (i.e., the transplantation of animal organs, tissue and cells into human beings) has been used to overcome the shortage of human donor material. The use of porcine organs (especially, islets of Langerhans) for transplantation appears to be the most promising option, given in particular, that the pig most closely resembles humans with regard to antigenic composition of tissues [6, 8, 14]. In this connection, it seems important to consider the potential for using porcine cornea as a xenograft in humans. We should give attention to the fact that after decellularization, the xenograft should be similar to the native human cornea with regard to immunological characteristics, biocompatibility, mechanical strength and transparency, thus creating an optimal micro-environment for subsequent migration of stromal and epithelial cells [8, 10, 15-17]. Therefore, developing and enhancing methods for decellularization of donor corneas for the development of bioengineered corneal models with the following keratoplasty is a task to be tackled by experimental and clinical ophthalmologists [5-7, 11, 16-17]. The purpose of this experimental animal study was to investigate the clinical efficacy of intralamellar keratoplasty with corneal stromal substitutes (CSS) developed using different decellularization methods. Materials and Methods Corneas were excised from enucleated pig eyes, and, to develop acellular CSSs, five methods of decelluarization of pig corneas were used that were different in the time and conditions of incubation with detergents (0.5% sodium dodecyl sulphate or TRITON X-100) and proteolytic enzymes (like 0.1% papain) [6]. In addition, an ultrasonic apparatus of type СD 3800 А (power, 50 W) was used to further improve the removal of cells and acellular corneal debris. A total of 40 Chinchilla rabbits (80 eyes; weight, 2.5–3 kg) were used in the experimental study. They were divided into 5 groups with different CSS versions. Preoperatively, animals were anesthetized with thiopental sodium (1 g/kg, intravenously). In each rabbit, the monocular IKP was carried out [1, 18] with one of the five CSS versions in the operating room setting. Postoperatively, antimicrobial and anti-inflammatory therapy was instituted. Animals were examined every other day for 30 days postoperative. This included fluorescein staining to assess corneal epithelial status. In addition, the following was assessed ophthalmoscopically: conjunctival discharge, conjunctival hyperemia, state of the CSS, state of the host cornea, and, at late follow-up examinations, graft acceptance and whether graft-versus-host disease was present [6]. The following items related signs of to were scored 0 to 3: conjunctival discharge (0, no discharge; 1, mild mucous discharge; 2, severe mucous discharge; 3, purulent mucous discharge), conjunctival hyperemia (0, pale pink conjunctiva as per physiological norm; 1, mild bulbar conjunctival hyperemia; 2, moderate bulbar conjunctival hyperemia; 3, severe bulbar conjunctival hyperemia), corneal graft edema (0, no edema and the whole cornea is transparent; 1, local corneal epithelial edema at inflammation site; 2, local epithelial edema with involvement of superficial stromal layers; 3, local edema involving both superficial and deep stromal layers), corneal fluorescein staining (0, no local staining; 1, punctate corneal staining; 2, less than 3 mm2 area; 3, more than 3 mm2 area). Inflammatory infiltration was scored 0 to 4 (0, no infiltration; 1, one to three punctate subepithelial infiltrates; 2, multiple punctate subepithelial infiltrates (n > 3); 3, multiple subepithelial infiltrates of 1 mm at least; 4, local infiltration involving both superficial and deep stromal layers). Corneal graft opacity was scored 0 to 1 (0 = no opacity; 1 = presence of opacity). Location of inflammation in the cornea was scored 0 to 1 (1, central; 2, paracentral). Animals were operated on and euthanized (at 1 month postoperatively) in accordance with the General Ethical Principles for Animal Experiments approved by the Third National Congress on Bioethics (Kyiv, 2007), and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (European Treaty series no. 123). Box plots were used to display the clinical rabbit eye scores after IKP with different group-related CSS versions (Nos.1-5; Figs. 1-7).
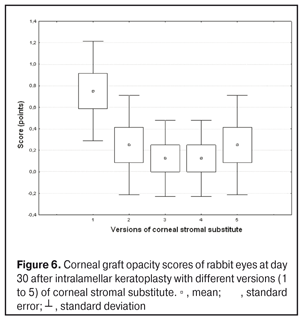
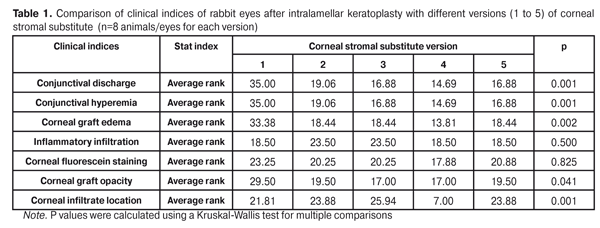
Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. The Kruskal Wallis test was used to compare the clinical scores at 1 month after IKP with a CSS within the groups. Pairwise comparisons among the groups were conducted using the Mann-Whitney test. Results and Discussion A Kruskal-Wallis test showed that there were statistically significant differences in the scores of conjunctival discharge, conjunctival hyperemia, corneal graft edema, corneal graft opacity, and location of corneal inflammation among the groups after IKP with a group-related CSS (Table 1). A Mann-Whitney test showed that, in group 1, the mean conjunctival discharge, conjunctival hyperemia and corneal graft edema scores were 4.3, 4.3 and 3.6-fold higher, respectively, than in group 2 (Figs. 1-7). There were statistically significant differences in the scores of conjunctival discharge (p = 0.01), conjunctival hyperemia (p = 0.01), and corneal graft edema (p = 0.005) between group 1 and group 2. In group 3, the mean conjunctival discharge, conjunctival hyperemia and corneal graft edema scores were 6.5, 6.5 and 3.6-fold lower, respectively, than in group 1 (Figs. 1-7). There were statistically significant differences in the mean scores of conjunctival discharge (p = 0.01), conjunctival hyperemia (p = 0.01), and corneal graft edema (p = 0.005) between group 3 and group 1. It should be noted that the mean scores of conjunctival discharge, conjunctival hyperemia, and corneal graft edema in group 4 were very low (0.13 points) (Figs. 1-7). In this group, the mean conjunctival discharge, conjunctival hyperemia and corneal graft edema scores were 12.5, 12.5 and 10.6-fold lower, respectively, than in group 1 (p = 0.001) (Figs. 1-7). In group 5, the mean conjunctival discharge, conjunctival hyperemia and corneal graft edema scores were 6.5 (p = 0.001), 6.5 (p = 0.001) and 3.6-fold (p = 0.005) lower, respectively, than in group 1 (Figs. 1-7). Although there were no statistically significant differences in the mean scores of conjunctival discharge, conjunctival hyperemia, and corneal graft edema between group 2 and group 3, the mean scores of conjunctival discharge and conjunctival hyperemia in the latter group were 1.5 and 1.5-fold, lower, respectively, than in the former group (Figs. 1-7). Eyes that received IKP with version 4 CSS showed 2.9-fold lower scores for conjunctival discharge, conjunctival hyperemia and corneal graft edema than those that received IKP with version 2 CSS, but these differences were also not significant (Figs. 1-7). In group 5, the mean conjunctival discharge and conjunctival hyperemia scores were 1.5-fold lower than in group 2, while the mean corneal graft edema score was the same in both groups (Figs. 1-7). In group 5, the mean inflammatory infiltration score was 2.9-fold lower, whereas the mean fluorescein staining and corneal graft opacity scores were 1.5 and 1.9-fold higher, respectively, than in group 3. No difference in conjunctival discharge, conjunctival hyperemia, and corneal graft edema was observed between the two groups (p > 0.05) (Figs. 1-7). In group 4, the mean conjunctival discharge, conjunctival hyperemia and corneal graft edema scores were 1.9, 1.9 and 2.9-fold lower, respectively, than in group 5 (Figs. 1-7). The mean corneal graft opacity score was better in eyes that received IKP with version 3 or 4 CSS (0.13 points) than in those that received IKP with version 2 or 5 CSS (0.25 points) or version 1 CSS (0.75 points). That is, in group 2 and group 5, the mean corneal graft opacity scores were 3-fold lower, than in group 1, whereas in group 3 and group 4, they were 5.8-fold lower, than in group 1. The mean corneal graft opacity scores in eyes that received IKP with version 3 or 4 CSS were 1.9-fold lower than in those that IKP with version 2 or 5 CSS. The differences in mean corneal graft opacity scores between group 1 and group 3, and between group 1 and group 4, were statistically significant (p < 0.015 and p < 0.015, respectively). Both Kruskal-Wallis test (Table 1) and Mann-Whitney test revealed no significant differences in the scores for inflammatory infiltration and corneal fluorescein staining among the five groups representing different CSS versions.
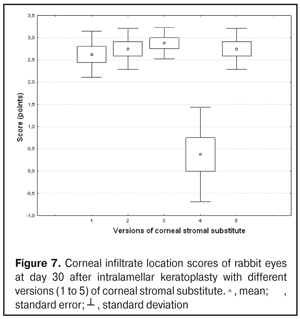
Eyes that received IKP with version 1, 4 or 5 CSS showed the best mean scores for inflammatory infiltration (0.13 points), and eyes that received IKP with version 4 CSS showed the best mean scores for corneal fluorescein staining (0.13 points) (Figs. 1-7). The inflammation location was paracentral in group 1 (2.63 points), group 2 (2.75 points), group 3 (2.87 points) and group 5 (2.75 points), whereas in group 4, the inflammation location score was 0.38 due to low extent of pathologic changes (Figs. 1-7). There was a significant intergroup difference in inflammation location score between the following pairs of groups: group 1 and group 4 (p = 0.003), group 2 and group 4 (p = 0.002), group 3 and group 4 (p = 0.001) and group 4 and group 5 (p = 0.002). Although numerous reports have been published on decellularization methods, many of them are related to experimental in vitro studies [9,10,19], and the CSSs developed in these studies do not always meet the requirements for mechanical strength, transparency and biocompatibility. Today, decellularized corneas are considered a better choice for keratoplasty than synthetic or semi-synthetic substitutes [5,9,10] because of higher mechanical indices and their potential for preserving the native matrix ultrastructure and intrinsic biological cues like growth factors [20]. The supply of human donor material is, however, limited by legal and ethical issues. If a currently available alternative to human donor cornea (like any xenograft development) [10,17,21] is to qualify for clinical use, a requirement which must be met is standardization of the most suitable decellularization methods. Moreover, potential cytotoxicity of various compounds used for decellularization of the cornea makes the requirements for decellularization conditions stricter. Although promising results have been reported by Pasyechnikova et al [18], Shafiq [22], Nasinnik [4] and Wilson [20] for the use of decellularized human corneal stromal pieces, this approach is limited by the issues related to donor material harvesting. The use of physical methods like high-hydrostatic pressurization for corneal tissue engineering has been demonstrated to provide decellularized cornea with good optical quality and without ultrastractural changes [23]; however, it may disrupt ECM ultrastructure [24]. In addition, post-pressurization immersion of decellularized cornea in glycerol for 1 hour resulted in restoration of the corneal optical properties to those of native corneas [25]. As opposed to Lee et al [26] and Yoeruek et al [27], we used 1) a combination of proteolytic enzymes with ultrasound pretreatment of corneal stroma for improved decellularization of porcine cornea, and 2) intralamellar keratoplasty to assess the developed corneal stromal substitutes. In our studies, the use of xenotransplantation and particular decellularization methods enabled us to solve the issues related to donor material harvesting and biocompatibility. We performed IKP with five different versions of CSS in eyes of experimental animals, and thereafter compared clinical rabbit eye indices among the groups with different CSS versions. This enabled us to determine a decelluarization method for the development of CSS that is adequately strong, relatively simple to process, and has a low production cost compared with prototypes. Conclusion
The lowest clinical scores for conjunctival discharge, corneal graft edema, inflammatory infiltration, corneal graft opacity and corneal fluorescein staining (0.13 points) were noted in rabbit eyes that underwent intralamellar keratoplasty with a version 4 corneal stromal substitute. Location of inflammation in the cornea was found to be paracentral in eyes that improved clinically after IKP with version 1, 2, 3 or 5 CSS, and tended to be central (0.38 points) in those that underwent IKP with version 4 CSS. Therefore, the least apparent reaction was noted in the rabbit eyes that underwent IKP with a version 4 CSS, which makes this CSS promising for further investigations in pre-clinical and clinical studies. References
|