J.ophthalmol.(Ukraine).2022;6:59-61.
|
http://doi.org/10.31288/oftalmolzh202265961 Received: 16.03.2022; Accepted: 20.09.2022; Published on-line: 21.12.2022 Corneal macular dystrophy. Case Presentation. Leopoldo Garduño-Vieyra, Bruno Flores Escobar, Isabel De la Fuente Batta Clínica Oftalmología Garduño Irapuato, Guanajuato (México) E-mail: contacto@oftalmologiagarduno.com TO CITE THIS ARTICLE: Leopoldo Garduño-Vieyra, Bruno Flores Escobar, Isabel De la Fuente Batta. Corneal macular dystrophy. Case Presentation. J.ophthalmol.(Ukraine).2022;6:59-61. http://doi.org/10.31288/oftalmolzh202265961
Among the stromal corneal dystrophies corneal macular dystrophy is one of the most frequent. It is an autosomal recessive disorder linked to chromosome 16, in which a mutation occurs in the CHST6 gene, causing an alteration in keratan sulfate metabolism. This alteration produces extracellular deposits of glycosaminoglycans between the stromal lamellae of the cornea, as well as in the cytoplasm of the endothelial cells. Clinically, the presence of centrally predominant white-greyish focal stromal corneal opacities is observed in early stages. Symptoms begin between the second and third decade of life and consist of progressive decrease in visual acuity and photophobia. In this work, we present the clinical case of a 56-year-old male patient who came to the clinic due to progressive decrease in visual acuity and photophobia. On physical examination, multiple intrastromal macules, whitish in color, were found by biomicroscopy in both eyes that were accentuated in greater quantity in the central 5 mm of the cornea. According to the findings obtained in the examination, the diagnosis of corneal macular dystrophy is established. Key words: сorneal macular dystrophy, keratoplasty, CHST6, keratin sulfate
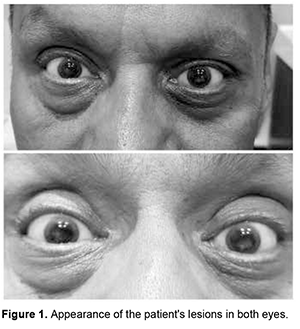
Introduction. The cornea is a complex structure that, in addition to fulfilling a protective mission, is responsible for three-quarters of the eye's optical power. The normal cornea lacks blood vessels; and it is nourished through tears and humor in its anterior and posterior portions, respectively. It is the body tissue with the highest nerve density, which is why certain disorders are associated with intense pain, photophobia and tearing [1]. Despite the variety of definitions that exist for the term “dystrophy”, it is used mainly to describe an inherited disease that affects cells, tissues or organs in isolation or in combination. In ophthalmology, the term “corneal dystrophy” has been used to refer to a group of hereditary corneal diseases, typically bilateral, symmetric, slowly progressive and not related to environmental or systemic factors; although there are exceptions to this definition. To understand corneal dystrophies, the anatomical classification of the latest IC3D update in 2015 is used, which is the most widely extended, dividing corneal dystrophies according to the affected stratum into: epithelial and subepithelial, epithelial-stromal (TGFBI dystrophies), stromal and endothelial. Within the stromal dystrophies we find macular dystrophy, Schnyder, François, speckled and pre Descemet. Other dystrophies that deserve mention, since they also affect the stromal portion, are the epithelial-stromal ones such as Reis-Bücklers, Thiel-Behnke, Lattice and its variants and the Granular type [2]. In the United States, a prevalence of 0.3 individuals per 250,000 inhabitants has been described, the most common being Macular, Granular and Lattice Corneal Dystrophy [3]. The present work aims to present a clinical case of an adult patient with stromal corneal opacities. Its physical examination, clinical diagnosis and treatment will be described, in addition to a brief review of the literature on this pathology. Case presentation We present the case of a 56-year-old male patient with no significant history who came to the clinic due to progressive decrease in visual acuity and photophobia of several years of evolution. The patient reports that he has attended multiple ophthalmological consultations and does not show any improvement after any treatment. (Figure 1)
Physical examination revealed visual acuity of RE: 20/80 and OS 20/100 with better corrected visual acuity (BCVA) of OD: 20/50 (+ 0.50 / -1.00x180 °) and OS: 20/80 (+ 0.50 / -1.00x180 °). Biomicroscopy revealed multiple whitish intrastromal macules that are accentuated in greater quantity in the central 5 mm of the cornea, a wide anterior chamber, a reflective pupil and a transparent lens. Eye strain is reported as 16 mm Hg. (Figure 2)
The fundus is seen with a red-orange background color and a well-applied retina in the posterior pole and periphery, papillae with well-defined edges with excavation of 4-tenths of a disc, emergence of normal vessels and present foveolar reflex; these features are present in both eyes. Based on the history, the patient's symptoms and the appearance of the lesions, a Macular Corneal Dystrophy is obtained as a diagnostic impression. Given that the patient does not meet the criteria for a penetrating keratoplasty for optical purposes, it is decided to establish the use of frame lenses with a transition effect to improve the patient's contrast sensitivity. Discussion Corneal Macular Dystrophy (CMD) belongs to the group of stromal corneal dystrophies, being the least frequent. It is characterized by being an autosomal recessive disorder in the CHST6 gene, presenting an alteration in keratan sulfate metabolism. Histologically it is characterized by extracellular deposits of glycosaminoglycans between stromal lamellae as well as in the cytoplasm of endothelial cells [4]. CMD is classified into 3 types, depending on the reactivity of keratan sulfate in the serum and the cornea to anti-keratan sulfate antibodies: type I (absence of keratan sulfate in cornea, serum and cartilage), type IA ( absent in serum, but the stroma shows immunoreactivity to keratin sulfate antibodies) and type II (presence of keratan sulfate in cornea, serum and cartilage, but with a synthesis less than 30% of normal). All histological types are clinically indistinguishable. CMD commonly manifests between the second and third decade of life, the main symptoms consist of photophobia, lacrimation and progressive deterioration of visual acuity. The lesions observed on examination are usually symmetrical, with a white-grayish appearance on the corneal surface as macular/spot opacities in the initial stages, which in late stages coalesce until reaching the limbus, deep stroma and endothelium [3]. The main differential diagnoses to be made are with all the types of dystrophies that affect the corneal stroma, already mentioned above. This can be carried out by the biomicroscopic characteristics of the lesions, through pathophysiological and genetic studies that associate the clinical characteristics [2]. The treatment of these patients with the following modalities has been described: - Phototherapeutic keratectomy with 193nm excimer laser and Mitomycin C: This technique has been used mainly in young patients, although in the studies consulted it has been seen that, during the follow-up of patients, there is again a decrease in visual acuity from the 5-60 months after treatment; It is concluded that it can be a useful treatment in young patients that could delay the performance of penetrating keratoplasty. - Deep anterior lamellar keratoplasty: It is another treatment alternative in patients without endothelial disease that has given optical and visual results comparable to full-thickness penetrating keratoplasty, while this technique preserves the density of corneal endothelial cells and eliminates the risk of endothelial rejection. Other studies have shown that it has a greater recurrence than penetrating keratoplasty and is more commonly seen in patients under 18 and over 30 years of age. - Penetrating keratoplasty: So far this is the preferred treatment modality. According to the results of Al-Swailem, the probabilities of graft survival are 98.1% at the first year, up to 74.1% at 15 years, being graft rejection more frequent when the patients submitted to this treatment modality are less than 40 years [5]. Alemán Suárez IO, Suárez Ojeda V, Oramas Armengol Y, et-al. indicate as a parameter for the indication of penetrating keratoplasty a reasonable expectation of significant visual improvement in the patient's condition and, in addition, a corneal condition that causes a better-corrected visual acuity lower than LogMAR 0.2 (20/100). The reason why it has been decided not to perform penetrating keratoplasty in our patient yet [6]. Conclusions Corneal macular dystrophy is an autosomal recessive disease that affects young individuals, it is characterized by abnormal corneal deposits that make it opaque, alter its structure and cause progressive visual decline. There are several treatments, which will depend on the corneal state, age and visual acuity of the patient.
References 1.Bowling B., Capítulo 6: Córnea. Kanski. Oftalmología clínica. Un enfoque sistemático. Sydney, Australia, ELSEVIER, 2016. 168 p. 2.Weiss JS., Møller HU., Aldave AJ., et al. IC3D Classification of Corneal Dystrophies-Edition 2. Cornea. 2015; 34: 117-159p. 3.Singh S., Das S., Kannabiran C., et al. Macular Corneal Dystrophy: An Updated Review. Current Eye Research. 2020; 1-6. 4.Gulias-Cañizo R, et al. Distrofia macular corneal: Características clínicas, histopatológicas y ultraestructurales. ARCH SOC ESP OFTALMOL. 2006; 81: 315-320. 5.Shields M., Craig JE., Souzeau E., Gupta A. Bilateral phototherapeutic keratectomy for corneal macular dystrophy in an adolescent: Case report and review of the literature. Ophthalmic Genetics. 2020; 41:4, 368-372. 6.Alemán Suárez IO., Suárez Ojeda V., Armengol Oramas Y., Hernández N. Queratoplastia penetrante con fines ópticos. Presentación de cuatro casos. Rev. Med. Electrón. 2020; 42:3, 1889-1899.
Information about authors and disclosure of information Corresponding author: Bruno Flores Escobar, brnfelm9@gmail.com. Authors’ contributions: Leopoldo Garduño-Vieyra – original idea and research; Bruno Flores Escobar – edition, head of capture; Isabel De la Fuente Batta – editind and writting. Funding information: This research has not received specific aid from public sector agencies, commercial sector or non-profit entities. Conflict of interest. The authors declare that they have no conflict of interest with this article.
|