J.ophthalmol.(Ukraine).2022;4:3-11.
|
http://doi.org/10.31288/oftalmolzh20224311 Received: 20.06.2022; Accepted: 18.07.2022; Published on-line: 24.08.2022 Sensitivity of peripheral blood lymphocytes to adrenaline and acetylcholine in patients with primary and recurrent posterior uveitis N. I. Khramenko, L. M. Velychko, O. V. Bogdanova, N. V. Konovalova, Iu. O. Zhuravok SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine" Odesa (Ukraine) TO CITE THIS ARTICLE: Khramenko NI, Velychko LM, Bogdanova OV, Konovalova NV, Zhuravok IuO. Sensitivity of peripheral blood lymphocytes to adrenaline and acetylcholine in patients with primary and recurrent posterior uveitis. J.ophthalmol.(Ukraine).2022;4:3-11. http://doi.org/10.31288/oftalmolzh20224311
Background: Posterior uveitis is a polyetiologic group of diseases with polymorphic clinical manifestations and a prolonged, chronic and recurrent course. In addition, it is commonly bilateral and often causes complications, loss of working capacity and early visual disability in young adults. It is an important task of current clinical ophthalmology to find biomarkers indicating the severity of inflammation and the likelihood of developing a recurrence of the initial process. Purpose: To determine the sensitivity of peripheral blood lymphocytes to adrenaline and acetylcholine in patients with primary and recurrent uveitis at different phases of the disease. Material and Methods: One hundred patients with idiopathic posterior uveitis were examined at different phases of the disease. The control group was composed of 16 healthy individuals of a similar age. The specific sensitivity of lymphocytes to neuromediators, adrenaline and acetylcholine (expression of T cell adrenergic and acetylcholine receptors) was assessed using our complex methodology (in conjunction with a parallel sampling method) for assessing the individual’s sensitivity to medicaments (biological regulators) which has been developed at Immunology laboratory, Filatov Institute of Eye Disease and Tissue Therapy. The method involves obtaining lymphocytes from an individual, culturing lymphocytes with examined drugs immunohistochemically, and use of a peroxidase anti-peroxidase method with monoclonal T-cell antibodies. Results: Absolute and relative adrenoreceptor and acetylcholine receptor expression levels in patients with posterior uveitis were 1.8-2.0 times and 1.6-2.4 times, respectively, higher in patients with posterior uveitis compared to controls. Absolute adrenoreceptor and acetylcholine receptor expression values were 12.6% and 31.7%, respectively, higher in the recurrent process during active inflammation compared to the period of remission. However, in patients with posterior uveitis, adrenoreceptor and acetylcholine receptor expression values in the period of remission were still higher than normal values. The odds of an increased relative adrenoreceptor expression and the odds of an increased relative acetylcholine receptor expression on peripheral blood lymphocytes were 67.5-fold higher and 23.6-fold higher, respectively, among patients with posterior uveitis compared to controls. The correlation of adrenoreceptor expression and acetylcholine receptor expression with absolute counts of T cells and T helpers was stronger than the correlation with B cells, which reflects to a larger extent the association with the cellular immunity and to a lesser extent the association with the humoral immunity. There was a moderate correlation of adrenoreceptor expression and acetylcholine receptor expression with cell subsets of markers of early lymphocyte activation CD5, autoimmune aggression CD25, intercellular adhesion CD54 and apoptosis CD95 (Fas/APO-1). Conclusion: The adrenoreceptor expression and acetylcholine receptor expression on peripheral blood lymphocytes may be used as a non-specific biomarker of an activated inflammatory process in posterior uveitis. Keywords: posterior uveitis, adrenaline, acetylcholine, lymphocyte activation biomarkers
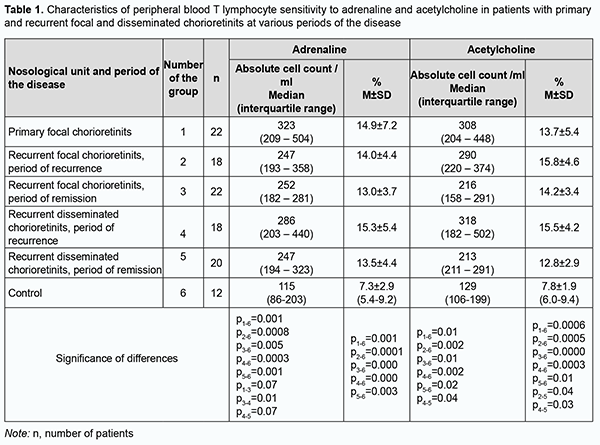
Introduction Posterior uveitis is a polyetiologic group of diseases with polymorphic clinical manifestations and a prolonged, chronic and recurrent course. In addition, it is commonly bilateral and often causes complications, loss of working capacity and early visual disability in young adults. Despite recent advances in research, establishing the etiology of uveitis and predicting complications and recurrence in patients with the disease remains a challenge. In this regard, the amount of inflammatory host response (a typical pathophysiological response) is extremely important. Too weak a response causes immunodeficiency and contributes to the dissemination of a causative agent, leading to the uncontrolled proliferation of transformed cells, while too strong a response causes tissue damage, fibrosis and immune disease, which is characteristic for autoimmune disorders (like rheumatoid arthritis, Crohn’s disease, multiple sclerosis, etc.), allergy, atherosclerosis and diabetes [1]. A number of observations and discoveries support the notion of the nervous system as an immunoregulatory system involved in immune responses. In particular, the peripheral nervous system, through neurotransmitters and neuropeptides, works in parallel with the hypothalamic-pituitary-adrenal and gonadal axis to modulate inflammatory events and maintain homeostasis [2]. Monoamines are a group of important bioactive substances of the central nervous system (CNS) that are considered to act as neuromodulators and regulate important functions, such as motor control, cognition, emotion, and memory processing. The major monoamines are serotonin, dopamine, and noradrenaline. Monoamines are also known to act beyond the CNS, and indirect effects of serotonin on NK cells through the regulation of monocytes by serotonin have been identified. Human immune cells express almost all dopamine receptors (DR). Among leukocytes, B cells and NK cells have the highest DR expression. Noradrenaline and subsequently adrenaline are synthesized from dopamine. They are found in serum at low concentrations, and can strongly increase during acute stress or exercise. Both noradrenaline and adrenaline bind to adrenergic receptors, but differ in their activation potencies. Adrenaline is a potent stimulator of beta adrenergic receptors (β-AR). NK cells express high levels of β2-AR but not β1-AR [3]. The role of adrenergic regulation in the function of NK cells has been studied [4]. β2-adrenergic receptors control lymphocyte recirculation through lymph nodes, contributing to the diurnal variation of lymphocyte recirculation, which is reflected in the magnitude of the adaptive immune response [5]. Though all lymphocytes have adrenergic receptors, differential density and sensitivity of adrenergic receptors on lymphocytes may affect responsiveness to stress among cell subsets. For example, natural killer cells have both high-density and high affinity β2-adrenergic receptors, B cells have high density but lower affinity, and T cells have the lowest density [6]. Therefore, there is a morphological basis for complex neuroimmune changes that mediate adaptive responses, eventually contributing to homeostasis. Neuropeptides are important players in immune function and chronic inflammation due to their chemoattractive capacity. Studies have demonstrated a key role of the sympathetic nervous system and its neurotransmitters in the regulation of chronic inflammatory conditions [7, 8]. The sympathetic nervous system is well known to play a critical role in regulating inflammatory conditions, and imbalanced sympathetic activity has been observed in rheumatoid arthritis [9]. We have previously found an association between the type of recurrence of herpetic keratitis and an increased tone of the sympathetic nervous system, with an impact of the autonomic sympathetic nervous system on a frequently recurring process being 27.2% greater than the impact on an infrequently recurring process [10]. In addition, we have examined the expression of adrenoreceptors and acetylcholine receptors on lymphocytes in peripheral blood in patients with anterior uveitis complicated by macular edema. We found that, in the period of recurrence, the expression of adrenoreceptors and acetylcholine receptors on lymphocytes in peripheral blood in the above patients was 32.7% and 25.2%, higher than in patients with uncomplicated uveitis [11]. It is an important task of current clinical ophthalmology to find biomarkers indicating the severity of inflammation and the likelihood of developing a recurrence of the initial process. The diagnostic evaluation using such markers would promote the development of therapy and early elimination of the process. The literature is scant on studies on the expression of adrenoreceptors and acetylcholine receptors on lymphocytes in the pathophysiological process in uveitis. The purpose of the study was to determine the sensitivity of peripheral blood lymphocytes to adrenaline and acetylcholine in patients with primary and recurrent uveitis at different phases of the disease. Material and methods The study cohort included 100 patients with idiopathic posterior uveitis who were treated at the Department of Ocular Inflammatory Disease and examined at the Department of Studies of Ocular Function and whose samples were obtained and processed at Immunology laboratory, Filatov Institute of Eye Disease and Tissue Therapy. Of these patients, 22 patients with focal chorioretinitis had a primary uveitic disease (with a duration of less than 3 months). A remission episode of recurrent uveitis was seen in 22 patients with focal chorioretinitis and 20 patients with disseminated chorioretinitis. A recurrent episode of uveitis was seen in 18 patients with focal chorioretinitis and 18 patients with disseminated chorioretinitis. The average patient age was 37.2 ± 1.5 years (mean ± standard deviation). The median duration of recurrent posterior uveitis was 2920 days (range, 1080 to 5110 days). The control group was composed of 16 volunteers (32 eyes) of a similar age without ocular disease or general medical condition. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Written informed consent was obtained from all participants. Patients underwent assessment of visual acuity, ophthalmoscopy, biomicroscopy, perimetry, and examination of the electrical sensitivity of the optic nerve and critical frequency of phosphene disappearance. Immunocytochemistry with monoclonal T-cell antibodies was used to determine lymphocyte subsets and lymphocyte activation markers. With this in mind, a 4-5-ml sample of heparinized blood was obtained from the cubital vein with a vacuum system, and twice diluted with 0.9% NaCl. To assess the specific reactivity of lymphocytes to adrenaline and acetylcholine, we employed our complex methodology (in conjunction with a parallel sampling technique) for assessing the individual’s sensitivity to medicaments which has been developed at Immunology laboratory, Filatov Institute of Eye Disease and Tissue Therapy [12]. The technique involves obtaining lymphocytes from an individual, culturing lymphocytes with examined drugs, and using an immunohistochemical peroxidase anti-peroxidase method with monoclonal T-cell antibodies. A summary of the method procedure is as follows. First, peripheral blood mononuclear cells from whole blood are separated through density gradient centrifugation using Ficoll separating solution with density of 1.077 g/mL and washed twice to obtain lymphocyte suspension. Second, (a) lymphocyte cell suspension (0.05 ml) is mixed with NaCl 0.9% (0.05 ml); (b) lymphocyte cell suspension (0.05 ml) is mixed with adrenaline 0.18% (0.05 ml; sterile solution, ready for use, manufactured by JSC Darnytsia, Kyiv, Ukraine); and (c) lymphocyte cell suspension (0.05 ml) is mixed with acetylcholine chloride 0.1% (0.05 ml; sterile solution, manufactured by Sinbias LLC, Kyiv) (dry substance with diluted with physiological saline); and these three mixture samples are incubated in parallel at 37оС for one hour. The protocols for concentrations of the used solutions have been developed previously by others [13]. Thereafter, T cells (CD 3) are determined immunohistochemically using a routine method [12, 14] with monoclonal T-cell antibodies. CD3 counts were determined for study samples (with adrenaline and acetylcholine) and control samples (with physiological saline). Absolute and relative expression of intercellular adhesion marker CD54 (ICAM-1), CD25, CD 5 and CD 95 in the peripheral blood lymphocytes were determined. Peripheral blood samples from the cubital vein were collected using a disposable vacuum system. Molecular markers of lymphocyte activation were assessed immunohistochemically using monoclonal T-cell antibodies [12, 14]. CD54 (ICAM-1) is a glycoprotein expressed on the cell surface of numerous cell types, has a molecular weight of 60-114 KDa, contains five Ig-like domains, and is a member of the immunoglobulin (Ig) superfamily. It is expressed on the cell surface of immune, endothelial and epithelial cells, induced by various inflammatory cytokines (interleukin-1-beta (IL-1β), interferon gamma (IFNγ) or tumor necrosis factor alpha (TNFα)), acts as an adhesion molecule and signal receptor in different types of cells to cause inflammatory reactions and enable inflammation and healing. CD25 is a member of the superfamily of protein receptors, a 55 kD glycoprotein and the alpha subunit of the interleukin (IL)-2 receptor. It is expressed on the cell surface of developing and activated T-lymphocytes, activated B-lymphocytes, NKT cells, monocytes and macrophages. Its biological value is in regulating proliferation and differentiation; supporting clonal balance of lymphocytes; and preventing an excessive immune response. Changes in CD25 expression can develop in inflammation of any etiology. CD25 molecule is an early marker of lymphocyte activation and reflects the capacity of lymphocytes for differentiation [15, 16, 17]. CD5 is a member of the superfamily of protein receptors expressed on T and B lymphocytes, a crucial immunomodulator both under homeostatic and inflammatory conditions, and is considered a marker of autoimmune aggression. It has been suggested that CD5 may regulate the generation and function of nTreg and iTregs, and that it plays a physiological role in Breg homeostasis [18]. Fas (APO-1, CD95) is a type I integral membrane protein with a molecular weight of 45 kDa, a member of the tumour necrosis factor (TNF) receptor family, is highly expressed on activated T and B cells. In addition, Fas ligand is capable of inducing apoptosis of Fas positive cells. Studies have shown high levels of soluble Fas in the serum in some infectious and autoimmune diseases and aging [19]. Parametric and non-parametric statistical methods were employed for analysis. Spreadsheets and STATISTICA 8.0 (StatSoft, Tulsa, OK) software were used for data collection, processing, visualization and analysis. The Kolmogorov-Smirnov test was performed to determine normality of data. Mean, standard deviation (SD) and 95% confidence interval (CI) values were calculated for normally distributed data. The Student t test was used to compare mean values of normally distributed numerical variables. Non-normally distributed data were described using median (Med) and interquartile range (IQR) and compared using Mann–Whitney U test. Nominal data were described using numbers with percentages. The level of significance p ≤ 0.05 was assumed. Spearman and Pearson correlation analyses were used for non-parametric and parametric data respectively. The STATISTICA 8.0 calculator was used to determine the significance of difference between two correlation coefficients. Odds Ratio (OR) was used as a quantitative measure of effect for the comparison of relative characteristics and defined as the ratio of the odds of an event occurring in the group affected by the risk to the odds of it occurring the control group. The 95% CI was calculated to project the OR values for the population. An association between the factor and the outcome was statistically significant if the confidence interval was above or below 1.00. Results Median and IQR values for adrenoreceptor expression on peripheral blood lymphocytes in the control group were 115 cell/µL and 86-203 cell/µL, respectively, which was significantly and 2.1-2.8 times lower than in patients with primary and recurrent posterior uveitis (Table 1). The relative adrenoreceptor expression on peripheral blood lymphocytes in the control group was 7.3 ± 2.9%, which was significantly and 1.8-2.0 times lower than in patients with posterior uveitis (Table 1).
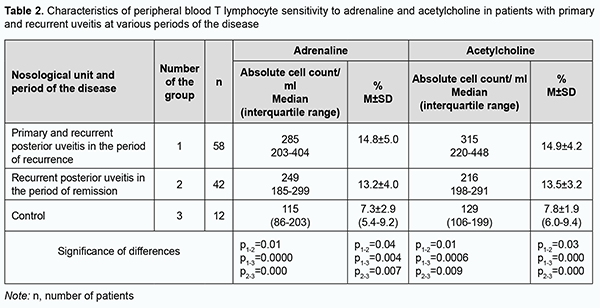
Median and IQR values for acetylcholine receptor expression on peripheral blood lymphocytes in the control group were 129 cell/µL and 106-199 cell/µL, respectively, which was significantly and 1.7-2.4 times lower than in patients with primary and recurrent posterior uveitis (Table 1). The relative acetylcholine receptor expression on peripheral blood lymphocytes in the control group was significantly and 1.6-2.0 times lower than in patients with primary and recurrent posterior uveitis (Table 1). There was a clear tendency to increased adrenoreceptor and acetylcholine receptor expression on peripheral blood lymphocytes in the period of apparent inflammation, and a tendency to decreased adrenoreceptor and acetylcholine receptor expression on peripheral blood lymphocytes in the period of remission (Table 1). Study patients with posterior uveitis were divided into a group of primary and recurrent posterior chorioretinitis in the period of recurrence (active inflammation) and a group of recurrent posterior uveitis in the period of remission (Table 2). In the active inflammation group, absolute and relative adrenoreceptor expression values were 12.6% (р=0.01) and 10.8% (р=0.04), respectively, higher, and absolute and relative acetylcholine receptor expression values, 31.7% (р=0.01) та 9.4% (р=0.03), respectively, higher, than in the group of recurrent posterior uveitis in the period of remission (Table 2).
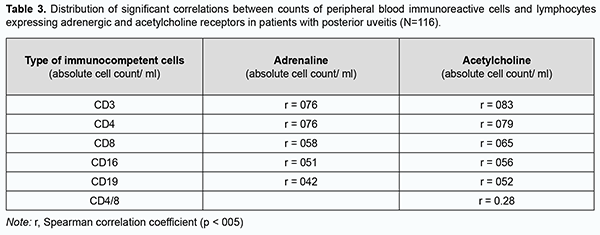
In patients with posterior uveitis, the association with a high relative adrenoreceptor expression (OR = 67.5; 95% CI, 8.0-565) was stronger than the association with a high relative acetylcholine receptor expression (OR = 23.6; 95% CI, 4.3-128). That is, the odds of an increased relative adrenoreceptor expression and the odds of an increased relative acetylcholine receptor expression on peripheral blood lymphocytes were 67.5-fold higher and 23.6-fold higher, respectively, among patients with posterior uveitis compared to controls. Correlation analysis was conducted to elucidate the essence of interrelated effects between adrenoreceptor expression and acetylcholine receptor expression on peripheral blood lymphocytes and immunocompetent cells (Table 3). Spearman correlation coefficient was calculated as a measure of association between numerical variables. Table 3 shows correlations of the number of immunocompetent cells with adrenoreceptor expression and acetylcholine receptor expression.
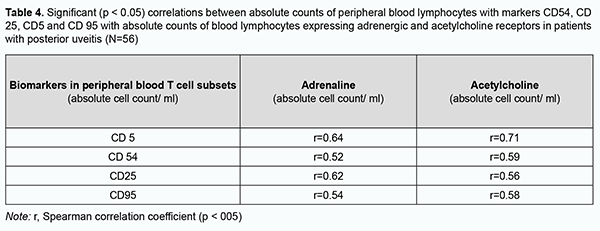
Correlation power was interpreted by evaluating the significance of difference in correlation coefficients. The correlation of adrenoreceptor expression with T cell (СD3+) count and T helper (СD4+) count (р = 0.006) was stronger than the correlation of adrenoreceptor expression with T suppressor (cytotoxic СD19+) count as a measure of humoral immunity (Table 3). The correlation of acetylcholine receptor expression with T cell (СD3+) count and T helper (СD4+) count (р = 0.01) was stronger than the correlation of adrenoreceptor expression with T suppressor (cytotoxic СD19+) (Table 3). We assessed correlations of cell count with adrenoreceptor expression, acetylcholine receptor expression with CD54, CD25, CD5 and CD95 lymphocyte subset counts (Table 4). There was a direct correlation of adrenoreceptor expression and acetylcholine receptor expression with СD5, СD54, СD25, and СD95 lymphocyte subsets with monoclonal T-cell antibodies. There was no difference in correlation power (as assessed by correlation coefficients) between adrenoreceptor and acetylcholine receptor expression with СD5, СD54, СD25, and СD95 lymphocyte subsets with monoclonal T-cell antibodies (Tab. 4), that is, all the markers were activated to the same extent with an increase in adrenoreceptor and acetylcholine receptor expression.
Therefore, with activation of inflammation in patients with posterior uveitis, absolute adrenoreceptor and acetylcholine receptor expression values increased by 12.6% and 31.7%, respectively, and relative adrenoreceptor and acetylcholine receptor expression values increased by 10.8% and 9.4%, respectively. There was a direct correlation of adrenoreceptor expression and acetylcholine receptor expression with absolute counts of T cells, T helpers, T suppressors and B cells. The correlation of adrenoreceptor expression and acetylcholine receptor expression with absolute counts of T cells and T helpers was stronger than the correlation with B cells, which reflects to a larger extent the association with the cellular immunity and to a lesser extent the association with the humoral immunity. Markers of early lymphocyte activation CD5, autoimmune process CD25, intercellular adhesion CD54 and apoptosis CD95 (Fas/APO-1) increased to the same extent with an increase in adrenoreceptor expression and acetylcholine receptor expression. Discussion “Immunological homeostasis” is essential for health, because insufficient or excessive activity in the immune systems causes disease. Humoral and cellular immune response activation protects damaged tissue from invasion, clears it from detritus, and initiates wound healing mechanisms and reparation. Today, life requires that the immune response to microbes and pathogens is maintained within a carefully balanced response range between dual threats: insufficient immunity, which would enable the pathogens to prevail, and excessive immunity, which can kill or impair the host, directly. Major advances in twentieth-century neuroscience and immunology revealed that neuronal circuits maintain homeostasis during immune responses. Homeostatic control of immune responses by neural reflex circuits occurs in a time frame that operates extremely fast relative to humoral and cell-trafficking mechanisms. Afferent reflex arcs sense pathogenic molecules, cytokines, and other products of infection and cell injury, thereby activating action potentials that travel rapidly, specifically, and directionally. These incoming neural signals to brainstem nuclei stimulate efferent action potentials that travel to the principal organs of the immune system, including the spleen, lymph nodes, and reticuloendothelial organs. Arrival of action potentials in these innervated immune tissues culminates in release of neurotransmitters that interact with specific receptors expressed by monocytes, macrophages, lymphocytes, and other cells of the innate and adaptive immune systems. There are increasingly available studies on the inflammatory reflex, the neural circuit composed of afferent and efferent neurons that travel in the vagus nerve to regulate immunity. The two arms of the autonomic nervous system typically act to balance arousal (sympathetic: “fight or flight”) with homeostasis (parasympathetic: “rest and digest”). There is no parasympathetic innervation in lymphoid organs, indicating that adrenergic signaling is a principal means of communication between the nervous system and the immune system. Adrenergic stimulation results in a rapid release of leukocyte subsets into the circulation [20]. Most mature leukocytes spend much of their cellular lives trafficking around the body with the goal of identifying and eradicating microorganisms and malignant cells. Soluble and cell-associated signals direct leukocyte trafficking, tissue entry, and migration within tissues. These include chemokines, cytokines, and adhesion molecules expressed by vasculature, tissues, and other immune cells. In addition to these well-defined signals, neurotransmitters produced by the nervous system and adrenal glands can also impact leukocyte migration and functions. Both leukocyte-intrinsic and -extrinsic pathways are influenced by neural signals to direct migration. Investigation of the effects of neurotransmitters on leukocytes in the blood, in particular the catecholamines noradrenaline (also known as norepinephrine) and adrenaline (epinephrine), spans more than a century, providing clear evidence of their impact on systemic leukocyte trafficking [21, 22]. Though all lymphocytes have adrenergic receptors, differential density and sensitivity of adrenergic receptors on lymphocytes may affect responsiveness to stress among cell subsets. For example, natural killer cells have both high-density and high affinity β2-adrenergic receptors, B cells have high density but lower affinity, and T cells have the lowest density [6]. Lymphocytes express most of the cholinergic components found in the nervous system, including acetylcholine (ACh), choline acetyltransferase (ChAT), high affinity choline transporter, muscarinic and nicotinic ACh receptors (mAChRs and nAChRs, respectively), and acetylcholinesterase. Stimulation of T and B cells with ACh or another mAChR agonist elicits intracellular Ca2+ signaling, increased nitric oxide synthesis and IL-2-induced signal transduction [23]. In addition, under inflammatory conditions, lymphocytes have been shown to express choline acetyltransferase and produce ACh. Although widely studied as a neurotransmitter, T cell-derived ACh has recently been reported to play an important role in regulating immunity and stimulate vasodilation. Vasodilation is critical for immune responses and is one of the hallmarks of inflammation facilitating the entry of immune cells into infected tissues. It has been shown that the enzyme choline acetyltransferase, which catalyzes the rate-limiting step of ACh production, is robustly induced in both CD4+ and CD8+ T cells during lymphocytic choriomeningitis virus infection in an IL-21-dependent manner. Deletion of choline acetyltransferase within the T cell compartment in mice ablated vasodilation in response to infection, impaired the migration of antiviral T cells into infected tissues, and ultimately compromised the control of chronic lymphocytic choriomeningitis virus infection [22, 24]. Therefore, the current literature highlights the role of neural control of the immune response to enable its speed, specificity and selectivity, the role of adrenergic and cholinergic regulation of the immune response under inflammatory conditions, and functions and cellular response to major neurotransmitters that can regulate migration, proliferation, differentiation and cooperation of immunocompetent cells. The role of neural control and neurotransmitters in the development of immune response in eye disease has been poorly studied. In the current study, we determined the levels of adrenoreceptor and acetylcholine receptor expression on peripheral blood T cells of healthy volunteers and patients with primary and recurrent posterior uveitis (focal and disseminated chorioretinitis). Absolute and relative adrenoreceptor and acetylcholine receptor expression levels in patients with posterior uveitis were 1.8-2.0 times and 1.6-2.4 times, respectively, higher in patients with posterior uveitis compared to controls. Absolute adrenoreceptor and acetylcholine receptor expression values were 12.6% and 31.7%, respectively, higher in the recurrent process during active inflammation compared to the period of remission. However, in patients with posterior uveitis, adrenoreceptor and acetylcholine receptor expression values in the period of remission were still higher than normal values. We found that the odds of an increased relative adrenoreceptor expression and the odds of an increased relative acetylcholine receptor expression on peripheral blood lymphocytes were 67.5-fold higher and 23.6-fold higher, respectively, among patients with posterior uveitis compared to controls. In addition, the correlation of adrenoreceptor expression and acetylcholine receptor expression with absolute counts of T cells and T helpers was stronger than the correlation with B cells, which reflects to a larger extent the association with the cellular immunity and to a lesser extent the association with the humoral immunity. Moreover, there was a moderate correlation of adrenoreceptor expression and acetylcholine receptor expression with cell subsets of markers of early lymphocyte activation CD5, autoimmune aggression CD25, intercellular adhesion CD54 and apoptosis CD95 (Fas/APO-1) increased to the same extent with an increase in adrenoreceptor expression and acetylcholine receptor expression. Therefore, in our opinion, the adrenoreceptor expression and acetylcholine receptor expression on peripheral blood lymphocytes may be used as a non-specific biomarker of an activated inflammatory process in posterior uveitis.
References 1.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335(6071):936-941. 2.Quatrini L, Vivier E, Ugolini S. Neuroendocrine regulation of innate lymphoid cells. Immunol Rev. 2018 Nov;286(1):120-36. 3.Capellino S, Claus M, Watzl C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cellular & molecular immunology. 2020 Jul;17(7):705–11. 4.Tarr AJ, Powell ND, Reader BF, et al. beta‐Adrenergic receptor mediated increases in activation and function of natural killer cells following repeated social disruption. Brain Behav Immun. 2012 Nov;26(8):1226-38. 5.Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med. 2016 Nov 14;213(12):2567-74. 6.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004 Jul;130(4):601-30. 7.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009 Feb;10(2):195-202. 8.Härle P, Straub RH, Wiest R, Mayer A, Schölmerich J, Atzeni F, et al. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic-pituitary-adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann Rheum Dis. 2006 Jan;65(1):51-6. 9.Ribeiro-da-Silva M, Vasconcelos DM, Alencastre IS, Oliveira MJ, Linhares D, Neves N, et al. Interplay between sympathetic nervous system and inflammation in aseptic loosening of hip joint replacement. Sci Rep. 2018 Oct 30;8(1):16044. 10.Khramenko NY, Ponomarchuk VS, Haydamaka TB, Drozhzhyna HY. [Features of the state of the autonomic nervous system and its influence on the regional hemodynamics of the eye in patients with different nature of the course of recurrent herpetic keratitis]. Oftalmol Zhurn. 2013;6:5-11. Russian. 11.KhramenkoN, Usov V, Velychko L, Konovalova N, Bogdanova O. Level of adrenoreception and acetylcholine reception on lymphocytes in peripheral blood in patients with anterior uveitis complicated by macular edema. DOG online. 2021. Abstract number A-1213-0052-00519. 12.Velychko L, Bogdanova O. Patent № 103483.[ A method for studying the receptor-modifying effect of pharmacological immunotropic drugs on markers of cell activation]. 2015, Bul No. 24. Ukrainian. 13.Dehtyarenko TV, Makulʹkyn RF. [Biogenic stimulants and immunoreactivity]. In 2 volumes, volume 2. Odessa: Mayak; 1997. Russian. 14.Hluzman DF, Sklyarenko LM, Nahornaya VA, Kryachok YA. [Diagnostic immunocytochemistry of tumors]. Kyiv:Morion; 2003. Russian 15.Litvinova LS, Gutsol AA, Sokhonevich NA, Kofanova KA, Khaziakhmatova OG, Shupletsova VV, et al. [Basic surface markers of functional activity T-limphocytes]. Medical Immunology. 2014;16(1):7-26. Russian. 16.Bui TM, Wiesolek HL, Sumagin R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 2020 Sep;108(3):787-99. 17.Sakamoto T, Takahira K, Sanui H, Kohno T, Inomata H. Intercellular adhesion molecule-1 on rat corneal endothelium in experimental uveitis. Exp Eye Res. 1993 Feb;56(2):241-6. 18.Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol. 2011 Jun;23(3):310-8. 19.Sugita S, Taguchi C, Takase H, Sagawa K, Sueda J, Fukushi K, et al. Soluble Fas ligand and soluble Fas in ocular fluid of patients with uveitis. Br J Ophthalmol. 2000 Oct;84(10):1130-4. Crossref PubMed 20.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313-35. 21.Nourshargh, S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014 Nov 20;41(5):694-707. 22.Mueller SN. Neural control of immune cell trafficking. J Exp Med. 2022 Mar 7;219(3):e20211604. 23.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003 Dec 26;74(6):675-96. 24.Cox MA, Duncan GS, Lin GHY, Steinberg BE, Yu LX, Brenner D, et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science. 2019 Feb 8;363(6427):639-44.
Disclosures Received 20.06.2022 Accepted 18.07.2022 Corresponding Author: Khramenko Nataliia, e-mail: khramenkon@gmail.com Disclaimer: the opinions presented in the article are solely the author's, they do not represent the official position of the institution. Sources of Support: There are no external sources of funding. Conflict of Interest Statement. The authors certify that there is no conflict of interest that could influence their opinion regarding the subject matter or materials described and discussed in this article.
|