J.ophthalmol.(Ukraine).2022;4:75-81.
|
http://doi.org/10.31288/oftalmolzh202247581 Published on-line: 24.08.2022 STANDARDS OF GLAUCOMA MEDICAL CARE
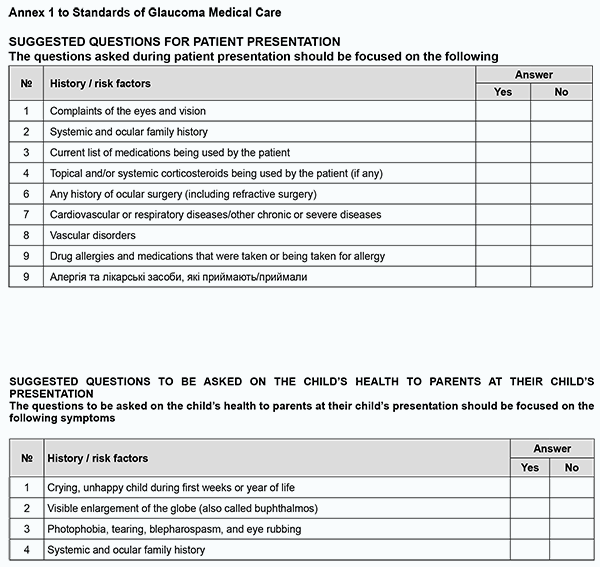
TO CITE THIS ARTICLE: Standards of glaucoma medical care. J.ophthalmol.(Ukraine).2022;4:75-81. http://doi.org/10.31288/oftalmolzh202247581 General International Classification of Diseases, 10th Revision (ICD-10) code for glaucoma = H40-H42 Developed by: Z.F. VeselovskaAcademician of NAMS of Ukraine, Dr Sc (Med), Professor and Chair of Surgical Disease Department No.2 at the Kyiv Medical University, a private higher education establishment, and President of the Ukrainian Glaucoma Society Paul HarasymowyczMD, Professor at the University of Montreal and Medical Director of the Montreal Glaucoma Institute and Bellevue Ophthalmology Clinics, Montreal, Canada Ilgaz YalvacDM, Professor of Ophthalmology at Yeditepe University School of Medicine; Director of the Istanbul Eye Center; President of the Turkish Glaucoma Society P.A. BezditkoDr Sc (Med), Professor and Chair of Ophthalmology at the Kharkiv National Medical University, and Vice President of the Ukrainian Glaucoma Society N.F. BobrovaDr Sc (Med), Professor, Head of the Department of Pediatric Eye Disorders, Filatov Institute of Eye Disease and Tissue Therapy, and Board Member of the Ukrainian Glaucoma Society N.M. VeselovskaDr Sc (Med), Professor of Surgical Disease Department No. 2 at Kyiv Medical University, a private higher education establishment, Head of the Eye Department at Kyiv Municipal Clinical Hospital No. 1, and Board Member of the Ukrainian Glaucoma Society S.K. DmytriievDr Sc (Med), Professor, Head of the Department of Cataract and Glaucoma, Filatov Institute of Eye Disease and Tissue Therapy, and Board Member of the Ukrainian Glaucoma Society N.G. ZavgorodniaDr Sc (Med), Professor and Chair of Ophthalmology at the Zaporizhzhia National Medical University, and Board Member of the Ukrainian Glaucoma Society S.O. RykovMember of NAMS of Ukraine, Dr Sc (Med), Professor and Chair of Ophthalmology at the National Healthcare University of Ukraine, and Board Member of the Ukrainian Glaucoma Society V.M. SerdiukDr Sc (Med), Professor and Chair of Ophthalmology at the Dnipro State Medical University, and Board Member of the Ukrainian Glaucoma Society I.V. ShargorodskaDr Sc (Med), Professor of the Department of Ophthalmology at the National Healthcare University of Ukraine, and Board Member of the Ukrainian Glaucoma Society O.M. LishchyshynaCand Sc (Med), Senior Researcher, Head of Medical Care Management Science Department at the Research and Practical Center of Preventive and Clinical Medicine of the State Administration Reviewers G. Iu. VengerDr Sc (Med), Professor and Chair of Ophthalmology at the Odesa National Medical University S.Iu. MogilevskyyDr Sc (Med), Professor of the Department of Ophthalmology at the National Healthcare University of Ukraine Standard 1. Communications and Multispecialty Management of Patients with Glaucoma Standard Statement There are clear mechanisms of communication (a) among glaucoma medical care providers and (b) between medical care providers and patients with glaucoma. Grounding Effective care-pathway-based communication systems conforming to the particular requirements of individuals are important for timely diagnostic evaluation, referral and treatment of patients. Patients with glaucoma should be assured that all healthcare specialists involved in their treatment communicate well with each other and them. Compulsory criteria 1.1. There must be developed and approved clinical pathways and written documents for coordinating and integrating primary, secondary and tertiary medical care for timely referral to secondary or tertiary medical care, diagnostic assessment and treatment of patients with glaucoma. 1.2. There must be an individualized documented plan of care incorporating information on patient’s diagnosis, treatment and management, and this plan has been agreed with the patient and is available to multidisciplinary care team members. 1.3. Patients and, if agreed, their family members or carers, must be provided with information on patient’s condition, treatment plan, follow-up and patient teaching the skills required for better care outcomes, and contacts for additional information and consulting, in easily understood and non-technical language. Standard 2. Diagnosing glaucoma Standard statement A comprehensive examination is performed to establish the diagnosis of glaucoma and/or detect any risk factors for glaucoma, the disease that can be seen at any age. Grounding The purpose of comprehensive examination is to establish the diagnosis of glaucoma. The mean IOP in adult populations is estimated at 15-16 mmHg, with a standard deviation of nearly 3.0 mmHg. An IOP of ≥ 21 mmHg is considered elevated. Patients with glaucoma tend to show diurnal variations in IOP and certain physiological parameters (e.g., ocular perfusion pressure), and these variations are more substantial than those in healthy individuals. Therefore, some patients might benefit from 24-h IOP and BP monitoring. Elevated IOP is the major risk factor for the development and progression of glaucoma. Unless for exceptional circumstances, a change-over between alternative types of IOP measurements is not recommended to prevent a disagreement between IOP measurements during a subsequent planned examination. The adults having signs of potential glaucoma or an associated condition on routine eye examination should have additional tests before being referred to diagnostic assessment services. Glaucomatous visual field defect and easily seen structural damage to the optic disc are signs of potential glaucoma. 2.1 Compulsory criteria 2.1.1. When taking history, attention must be given to symptoms and risk factors for the development of glaucoma as per Annex 1.
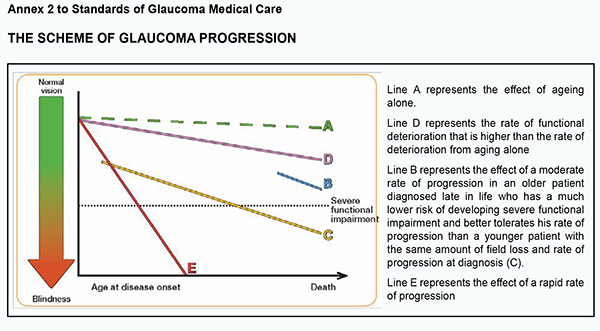
2.1.2. The patient’s IOP must be measured using Goldmann slit-lamp applanation tonometry or alternative techniques including ICare rebound tonometry, non-contact tonometry, dynamic contour tonometry, ocular response analyzer, Ocuton S tonometry, Tono-Pen tonometry, Maklakoff applanation tonometry or transpalpebral tonometry. 2.1.3. Anterior chamber angle landmarks are identified using direct or indirect gonioscopy. Gonioscopy must not be replaced by digital imaging of the anterior chamber angle. 2.1.4. A slit-lamp examination by the Van Herick technique is used to estimate the peripheral depth of the anterior chamber. 2.1.5. The fundus is examined using ophthalmoscopy or photography under short mydriasis to detect any glaucomatous optic disc changes and hemorrhages. 2.1.6. Perimetry is performed in the following phases in sequential order: 2.1.6.1. Assessing Visual Fields by Standard Automated Perimetry (SAP) or Non-conventional Perimetry 2.1.6.2. Interpreting Test Results (printouts, reliability indices, visual field indices, recording the visual field indices, summarizing diagnostic features (Glaucoma Hemifield test, Bebié curve, diagnosis based on clustered points, and assessing progression by event analysis, trend analysis, and number of tests)) 2.1.6.3. Staging of Visual Field Defects 2.1.6.4. Short-wave automated perimetry (SWAP) is not recommended in glaucoma due to a large measurement error. 2.1.7. Central corneal thickness (CCT) is measured to estimate IOP. The normal distribution of IOP is 540 ± 30 µm, with an increase or decrease in CCT resulting in an increase or decrease in IOP as assessed by applanation tonometry (≤ 2 mmHg per 50 µm of IOP). It is not recommended to “correct” IOP based on CCT. 2.1.8. Qualitative clinical examination is performed by comparing current and baseline photographs to detect progressive thinning of the neuroretinal rim, diffuse thinning or localized defects of the retinal nerve fiber layer (with a red-free (green) light or a short, narrow beam of bright white light at high magnification), optic disc hemorrhages, and changes in the position of the vessels at the optic disc with bending, bayoneting or baring of circumlinear vessels. Provocative tests for angle-closure are not recommended since even when negative they may not rule out the potential for angle closure. In addition, eyes with positive provocative tests may never develop angle closure. 2.1.8.1. A decision on establishing the diagnosis of and administering a treatment for glaucoma must not be made based only on the results of digital examination (artificial intelligence) since these results may only support the results of clinical assessment. 2.2. Desired criteria 2.2.1 Assessing changes in optic disc characteristics and neuroretinal rim width by qualitative imaging techniques (optical coherence tomography, Heidelberg retinal tomography, and scanning laser polarimetry). The optic cup to disc ratio is not reliable for diagnostic purposes and the detection of progression since it is highly variable in various sizes and shapes of the optic disc. Standard 3. Treating patients for glaucoma Standard Statement The treatment of glaucoma is aimed at lowering IOP and reducing the rate of visual field deterioration. Grounding The goal of glaucoma treatment is to maintain the patient’s visual function and related quality of life. Patients with early to moderate glaucoma damage have good visual function and modest reduction in quality of life, while quality of life is considerably reduced if both eyes have advanced visual function loss. The patient-centered principles must be adhered to while managing for glaucoma; patients with severe functional loss or younger patients with manifest disease should have more aggressive treatment and closer follow-up than patients with little or no risk, e.g., patients with ocular hypertension or elderly patients with early field loss and low IOP levels (please, see Annex 2).
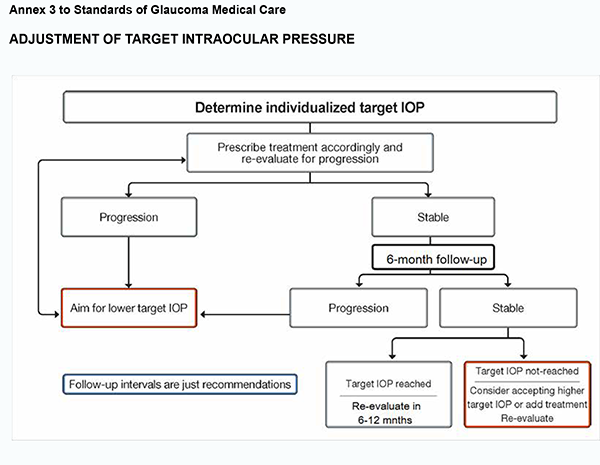
The rate of progression is an important factor that should be considered in determining the target IOP and intensity of treatment. Target IOP is the upper limit of the IOP estimated to be compatible with a rate of progression sufficiently slow to maintain vision-related quality of life in the expected lifetime of the patient. The treatment target is a compromise between reducing the risk of symptomatic vision loss and the side effects of therapy. Patient’s engagement in the treatment process, compliance and adherence to care is essential for effective IOP lowering and prevention of glaucoma progression. Compulsory criteria 3.1. Target IOP needs to be estimated separately for each eye of every patient. 3.1.1. In newly diagnosed patients, the target IOP is initially determined according to the stage of disease and the starting IOP, with the treatment goal being a specific IOP level or a percentage reduction. 3.1.2. Initial therapy may be with topical medication or selective laser trabeculoplasty in order to achieve a target IOP. 3.1.3. The target IOP is re-considered if a patient fails to attain a target IOP during follow-up or additional therapy is being administered, or the patient is being switched from one treatment to the other. If there are sufficient visual fields to judge the rate of progression, and this rate is sufficiently slow not to affect the patient’s quality of life, that the target IOP may be revised upward if the target IOP has not been met, or if the patient is on excessive therapy or is experiencing side effects (see Annex 3).
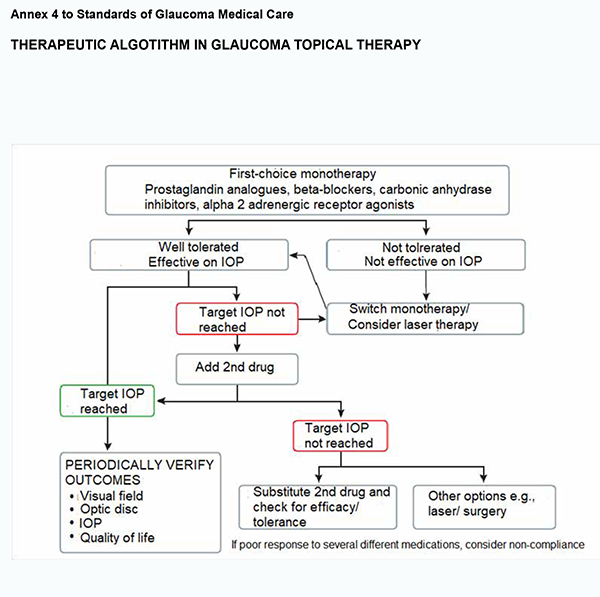
3.2. Most types of open angle glaucoma and many types of chronic close-angle glaucoma are initially treated with topical medications and occasionally oral osmotic. 3.2.1. Monotherapy is the first choice when initiating therapy. Prostaglandin analogues cause the greatest reduction in IOP and are considered most effective, followed by topical carbonic anhydrase inhibitors, non-selective beta-blockers, alpha adrenergic receptor agonists and selective beta-blockers. If the initial therapy reduces IOP to the target and is well tolerated, the therapy can be left unchanged, but the patient needs to be monitored with regular checking of endpoints (see Annex 4).
3.2.2. The selection of the topical therapy should take into account the pharmacological characteristics of the drug, the patient’s contraindications and patient’s general health condition. 3.2.3. In the absence of ocular surface disease and symptoms of allergy, a topical preservative-containing medication is used as the first-line treatment. In the presence of ocular surface disease (dry eye disease) or symptoms of allergy, a topical preservative-free medication is used as the first-line treatment or the patient needs to be urgently switched from a topical preservative-containing medication to a topical preservative-free medication. 3.2.4. If the initial therapy does not seem effective, with the target IOP not being reached, or the drug is not tolerated, one should switch to another therapy from the same class of drugs and then from another class of drugs. 3.2.5. If the first-line monotherapy is well tolerated and has effective IOP lowering, but has not succeeded in reaching the target IOP, the addition of the second drug (from another class of drugs like non-selective alpha adrenergic receptor agonists, parasympathomymetics (cholinergic drugs), or osmotics) or the use of fixed-combination therapy should be considered. 3.2.6. In cases with advanced glaucoma or juvenile glaucoma an IOP lower than 21 mmHg should be reached. 3.3. In patients with a very high IOP, surgical techniques (non-perforating deep sclerectomy, trabeculectomy, trabeculotomy, viscocanalostomy and canaloplasty) are used to maintain vision. Antimetabolites or other methods are used to prevent scarring of the filtration bleb. Sinus trabeculectomy remains the gold standard for microsurgery for primary open-angle glaucoma. It is not recommended to perform surgery on painful blind eyes with high IOP. 3.3.1. Many types of pediatric glaucoma are managed with early surgery. Although acute angle closure with or without glaucoma needs rapid laser or incisional surgery, medical treatment usually will be initiated as a first step. There is, however, no evidence that selective laser trabeculoplasty (SLT) is more effective than medication treatment for juvenile glaucoma. 3.3.2. Laser treatment (mostly with SLT) may be a suitable first option for patients with known intolerance or allergy to topical agents or suspected poor compliance. 3.3.3. Laser surgery treatment options include laser iridotomy, selective laser trabeculoplasty, laser iridoplasty, and cyclophotocoagulation. 3.3.4. When glaucoma surgery is indicated and there is a visually significant cataract, glaucoma surgery and minimally-invasive cataract extraction can be performed combined or sequentially. Standard 4. Monitoring for glaucoma Standard statement The patient with glaucoma needs to be monitored with regular assessment of the condition of the eyes and visual system. This should be done, particularly, during primary treatment for glaucoma for determining a target pressure. It is important that the target IOP is not a fixed value and may be adjusted with time, and, therefore, should be re-evaluated regularly, and, if required, adjusted, with its re-evaluation period depending on the visual function. Grounding Glaucoma is a chronic progressive disease that requires long-term continuous engagement of the patient with the recommendations proposed by the doctor. Some diagnostic and treatment recommendations that have been based on the results of a large and prolonged trial should be taken into account in particular cases. Target IOP should be re-evaluated regularly. In a newly diagnosed patient, the rate of progression is unknown, and target IOP is based on risk factors for progression. After sufficient follow-up, and with sufficient visual field test to tests to reliably determine the progression status, usually 2-3 years, the importance of the risk factors for decision-making decreases, importance of the measured rate of progression increases, and the RoP should be used to adjust the target IOP. Target IOP should be re-evaluated and modified accordingly when the progression of the disease is identified or when ocular or systemic comorbidities develop. The RoP should be used to adjust the target IOP, taking into account IOP levels over the observation period, life expectancy, and current levels of visual function damage. Patient’s engagement in the treatment process is essential for effective IOP lowering and prevention of glaucoma progression. This engagement is characterized by adherence to the glaucoma therapy that has been prescribed taking into account the pharmacological characteristics and side effects of the drug and the patient’s contraindications and general health condition. Compulsory criteria 4.1. Questions asked during monitoring are focused on the state of patient’s eyes, changes in this state, the patient’s understanding of the diagnosis and adherence to the current treatment plan, and checking the patient’s compliance. 4.2. There are two main approaches to computer-assisted visual field progression analyses. Progression event analyses seek to detect whether or not a statistically significant visual field change has occurred. Trend analyses aim to determine the visual field rate of progression, with at least five reliable tests to be performed for this purpose. 4.3. Newly diagnosed glaucoma patients should be tested with SAP three times per year during the first two years after diagnosis. If no progression is detected over the two years of perimetry monitoring, the tests can be performed less frequently. 4.4. After an individually specified period of follow-up (2-3 years), the RoP should be used to adjust the target IOP. The greater the RoP, the higher the target IOP. 4.5. Quantitative imaging of the optic disc, retinal nerve fiber layer and inner macular layers should be performed three times a year to detect glaucomatous progression during follow-up. 4.5.1. It is advantageous to follow patients using a consistent test pattern and strategy, in order to facilitate detection and quantification of progression. 4.5.2. In patients at a high risk of disease progression, a follow-up examination is performed three months after initial examination, with at least four more follow-up examinations performed over the two first follow-up years. References 1.[Terminology and Guidelines for Glaucoma]. European Glaucoma Society. 2018. PubliComm. 4th edition. Ukrainian. 2.Terminology and Guidelines for Glaucoma. European Glaucoma Society. 2020. PubliComm. 5th edition.
3.[Terminology and Guidelines for Glaucoma]. European Glaucoma Society. 2021. PubliComm. 5th edition. Ukrainian.
|