J.ophthalmol.(Ukraine).2022;3:39-44.
|
http://doi.org/10.31288/oftalmolzh202233944 Received: 27.01.2022; Accepted: 25.04.2022; Published on-line 15.06.2022 Influence of corvitin and metformin on biochemical changes in lacrimal glands of rats during water avoidance stress modeling Ye. K. Matsytska, O. Ye. Akimov, A. O. Mykytenko Poltava State Medical University; Poltava (Ukraine) TO CITE THIS ARTICLE:Matsytska YeK, Akimov OYe, Mykytenko AO. Influence of corvitin and metformin on biochemical changes in lacrimal glands of rats during water avoidance stress modeling. J.ophthalmol.(Ukraine).2022;3:39-44. http://doi.org/10.31288/oftalmolzh202233944 Background. Dry eye disease is a multifactorial condition, which is characterized by impairment of tear film formation. Lacrimal glands metabolism plays a critical role in dry eye disease. Emotional stress may impair lacrimal glands function. Purpose. We aimed to study production of nitric oxide from constitutive and inducible NO-synthases, activity of arginases and oxidative stress markers in lacrimal glands of rats during modeling of water avoidance stress (WAS) and its correction by metformin and corvitin. Material and methods. We concluded our experiment on 36 adult male rats of Wistar line weighing 190-240 g. Animals were divided into 6 groups consisting from 6 animals each, namely: control group, WAS group, group of correction by metformin (200 mg/kg) and group of correction by corvitin (10 mg/kg) during WAS modeling. And two drug-control groups. Results. WAS leads to increased activity of inducible NO-synthase, superoxide dismutase, catalase and concentration of MDA by 1.59, 1.93, 1.97 and 1.28 times respectively. Metformin and corvitin decreased activity of inducible NO-synthase by 8.25 and 8.5 times respectively, concentration of MDA decreased by 1.35 and 1.26 times respectively. Activities of superoxide dismutase did not change after introduction of metformin and corvitin. Metformin decreased catalase activity by 1.47 and corvitin increased it by 1.55 times. Production of superoxide dropped during WAS by 1.59 times and was increased to level below or equal that of control animals with introduction of metformin and corvitin. Conclusion. Increased activity of inducible NO-synthase during WAS is a possible reason of tissue damage in lacrimal glands of rats. Introduction of metformin or corvitin during WAS are an effective means for correction of tissue damage in lacrimal glands of rats due to their ability to lower increased inducible NO-synthase activity. Кey words: corvitin, metformin, lacrimal glands, water avoidance stress
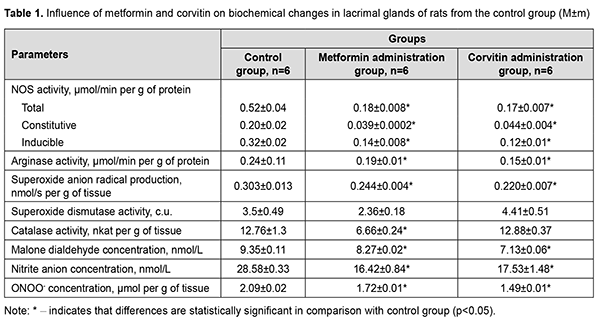
Introduction Dry eye disease is a multifactorial condition, which is characterized by impairment of tear film formation. Insufficient production of tears and low quality of tears are among main reasons of defective tear film formation. Dry eye disease can reach prevalence of 75% among the adults over 40 years old [1]. Its prevalence increases with age and chronic illness comorbidities, such as depression, diabetes and glaucoma [2]. In scientific literature, there are evidences of major role of oxidative stress in development of dry eye disease. The imbalance of reactive oxygen species (ROS) production and activity of antioxidant enzymes is a key factor in oxidative damage to ocular surface tissues [3]. Redox sensitive transcriptional factors also have a certain role to play in development of dry eye disease. For instance, erythroid-2–related factor 2 (Nfr-2) recognizes oxidative damage on cellular level and activates the transcription and translation of antioxidant enzymes, namely superoxide dysmutases (SOD) and catalase (CAT) [4]. However lacrimal glands also play important role in dry eye disease pathogenesis. Removal of lacrimal glands leads to activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in conjunctival tissue, with subsequent oxidative damage [5]. People suffering from emotional stress have increased chances of dry eye disease development [6]. Increased transcription of inducible NO-synthase (iNOS) gene in lacrimal glands may also contribute to dry eye disease formation, since concentration of iNOS is increased in tears obtained from Video Display Terminal workers [7]. Metformin has shown its potency in treatment of dry eye disease caused by Sjögren's syndrome due to its ability to alleviate inflammation of lacrimal glands [8]. This effect was observed due to inhibition of NF-κB activation and AMPK activation in lacrimal glands. Topical usage of quercetin also proved its effectiveness in treatment of dry eye disease [9]. However, topical influence may be only symptomatic treatment because it does not improve the state of lacrimal glands. Parenteral usage of water soluble form of quercetin (corvitin), developed and produced in Ukraine, may be a possible pathogenetic treatment of dry eye disease. Biochemical changes in lacrimal glands, including abovementioned production of reactive oxygen and nitrogen species, during emotional stress are still described insufficiently in scientific literature. Although there are evidences about positive influence of treatment of dry eye disease with medications influencing redox sensitive transcriptional factors, their impact on lacrimal glands during emotional stress is still unrevealed. The purpose of this work is to study production of nitric oxide from constitutive and inducible NO-synthases, activity of arginases and oxidative stress markers in lacrimal glands of rats during modeling of water avoidance stress (WAS) and its correction by metformin and corvitin. Material and methods We conducted our experiment on 36 adult male rats of Wistar line weighing 190-240 g. Animals were divided into 6 groups consisting of 6 animals each. First group (Control group). Animals of this group were placed on a platform (8×6 cm) in the middle of a plastic container with a diameter of 90 cm and a height of 50 cm without water. Rats stayed on the platform for 1 hour during 10 days. Additionally, these animals received an intraperitoneal injection of 0.1 ml 0.9% (w/v) sodium chloride solution and intragastrical injection of 1 ml of 0.9% (w/v) sodium chloride solution via feeding probe. Animals from second group (WAS group) were subjected to water avoidance stress as described by K. Yamamoto et al. [10]. Briefly, the rats were placed on a platform (8×6 cm) in the middle of a plastic container with a diameter of 90 cm and height of 50 cm filled with water of 25°C to 1 cm below the level of the platform. Rats avoided water by staying on the platform for 1 hour during 10 days [10]. Animals from third group on the background of WAS modelling received 200 mg/kg intragastrically 1,1-dimethylbiguanidine hydrochloride (metformin, TOV “Astrapharm”, Ukraine) each day [11]. Animals from fourth group on the background of WAS modelling received 10 mg/kg intraperitoneally corvitin, a quercetin complex with polyvinylpyrrolidine produced by ZAT NVTS “Borshchahivsʹkyy CPP”, Ukraine (dose was calculated in terms of quercetin) each day [12]. Two additional groups consisting of 6 animals each were formed (groups five and six). These groups were formed in order to evaluate an influence of medications (metformin and corvitin) on lacrimal glands without WAS interference. Metformin control group (group five) included animals, which were subjected to the same procedure as animals from the control group, but additionally received intragastrically 1,1-dimethylbiguanidine hydrochloride (metformin, TOV "Astrapharm", Ukraine) each day. Corvitin group (group six) followed the same procedure as mentioned above, but received 10 mg/kg intraperitoneally corvitin each day. Animals were removed from experiment under thiopental narcosis by drawing blood from right heart ventricle. All biochemical studies were conducted in 10% lacrimal gland tissue homohenate using Ulab 101 spectrophotometer. The assessment of total NO-synthases activity (E.C. 1.14.13), arginases activity (E.C. 3.5.3.1), concentration of nitrites (NO2-) and peroxinitrites (ONOO-) was performed as described in paper by O.Ye. Akimov and V.O. Kostenko [13]. The activity of inducible NO-synthase (E.C. 1.14.13.39) and constitutive forms of NOS (cNOS) was evaluated as described by A.M. Yelins’ka et al. in her work [14]. We evaluated the production of the superoxide radical anion (SAR) by estimating the concentration of diformazan, yielded in reaction between SAR and Nitroblue Tetrazolium [15]. The activity of superoxide dismutase (E.C. 1.15.1.1, SOD) was determined according to the recommendations proposed by O.S. Brusov [16]; the activity of catalase (E.C. 1.11.1.6, CAT) was assessed by the method of M.A. Korolyuk [17]. Protein concentration was determined by Biurette method. The concentration of free malondialdehyde (MDA) was assessed by method based on reactions of 1-methyl-2-phenylindole with malondialdehyde [18]. The results were tested for the data normality by the Shapiro-Wilk test. Then it was analyzed by non-parametric analysis by the Mann-Whitney test. The difference between the groups was considered statistically significant when p <0.05. For statistical studies we used Microsoft Office Excel and “Real Statistics” add in for Excel (developed by Charles Zaiontz). Data is represented as mean and standard error of mean (M±m). Results Introduction of metformin caused a decrease in total NOS activity 2.89 times, iNOS activity 2.29 times and cNOS activity in 5.13 times (Table 1). This change in NO production had led to decrease in superoxide anion-radical production by 19.5%. The activity of SOD did not change and activity of CAT reduced 1.92 times. MDA concentration and nitrite concentration in lacrimal glands reduced by 11.6% and by 42.55% respectively. Peroxynitrite concentration lowered by 17.7%. Arginase pathway of L-arginine cleavage decreased its activity by 20.8%.
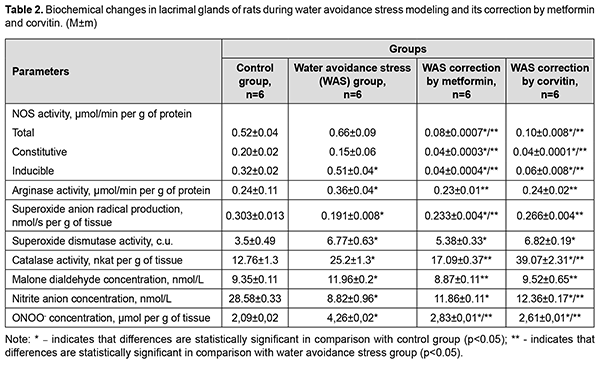
Corvitin introduction showed similar changes, but did not change antioxidant enzymes activity. Activity of iNOS and cNOS dropped by 2.67 times and 4.55 times respectively. Total NOS activity decreased by 3.06 times. Arginases activity dropped by 37.5%. MDA concentration and nitrite concentration in lacrimal glands reduced by 23.74% and by 38.7% respectively. Peroxynitrite concentration lowered by 28.7%. The results showed statistically significant a 1.59-fold increase in the activity of iNOS in the lacrimal glands of rats during WAS modeling compared with the control group of animals (Table 2). The total activity of NOS in the lacrimal glands of rats under conditions of WAS correction by metformin decreased by 8.25 times, the activity of cNOS decreased by 3.75 times and the activity of iNOS dropped by 12.75 times compared with WAS group. The total NOS activity in the lacrimal glands of rats under the conditions of WAS correction by corvitin decreased 6.6 times, the activity of cNOS lowered by 3.75 times and the activity of iNOS dropped by 8.5 times compared with WAS group.
The activity of arginases in the lacrimal glands of rats under WAS increased 1.5 times compared with the control. WAS correction by metformin and corvitin decreased arginase activity in the lacrimal glands of rats by 1.57 and 1.5 times respectively. The concentration of nitrites in the lacrimal glands of rats under WAS decreased by 3.24 times compared with the control group. The concentration of nitrites in the lacrimal glands of rats under conditions of WAS correction by corvitin increased 1.4 times compared with WAS group. Metformin did not have any statistically significant influence on nitrite concentration in the lacrimal glands of rats during WAS modelling. The baseline SAR production decreased by 1.59 times in the lacrimal glands of rats under WAS compared with the control group of animals. Baseline SAR production increased 1.22 times in the lacrimal glands of rats under conditions of WAS correction by metformin and 1.39 times under conditions of WAS correction by corvitin compared with WAS group. The activity of SOD in the lacrimal glands of rats under WAS increased 1.93 times compared to control group. Introduction of metformin and corvitin as means of WAS correction did not change the activity of SOD in the lacrimal glands of rats. Catalase activity in the lacrimal glands of rats under WAS increased 1.97 times compared to control group. Catalase activity in the lacrimal glands of rats under conditions of WAS correction by metformin decreased by 1.47 times compared with WAS group. Catalase activity in the lacrimal glands of rats under conditions of WAS correction by corvitin increased 1.55 times compared with WAS group. The concentration of MDA in the lacrimal glands of rats under WAS increased by 1.28 times compared with the control group. WAS correction by metformin and corvitin decreased MDA concentration in the lacrimal glands of rats by 1.35 and 1.26 times respectively. Discussion WAS led to intensification of lipid peroxidation (LPO) processes in lacrimal glands of rats as had been proven by an increase of MDA concentration. However, this increased LPO process has its own peculiarities. For instance, WAS decreased SAR production in lacrimal glands of rats, while increasing activity of SOD and CAT. Increased antioxidant activity should have decreased intensity of LPO processes, but we observed quite opposite situation. The reason for decreased SAR production and increased LPO may lie in increased peroxynitrite formation. In our study we established, that activity of iNOS is increased during WAS modeling. At the same time nitrite concentration is decreased in lacrimal glands of rats. Nitrite is usually formed in reaction of nitric oxide (NO) with oxygen (O2) present in tissues, while toxic peroxynitrite is yielded in reaction of abovementioned nitric oxide with superoxide anion radical (O2•-). Therefore, decreased SAR production and nitrite concentration in lacrimal glands of rats may be the result of increased peroxynitrite formation, which in turn may lead to increased LPO. Peroxynitrite can react with carbon dioxide (CO2) resulting in formation of highly reactive carbon trioxide (CO3•-) radical [19]. A certain limitation of our work is the fact, that concentration of nitrate anion (NO3-) was not evaluated. Nitrate being the second stage product of nitric oxide oxidation by oxygen usually expresses less potent chemical activity and is a metabolite to be excreted by kidneys to remove excessive nitric oxide formed by NO-synthases [20]. However, it can be reduced back to nitrites and even nitric oxide by nitrate-nitrite reductases [21]. Increased arginase activity may be considered as adaptive response to tissue damage. Arginase activity leads to formation of potent stimulators of tissue regeneration (putrescine, spermidine, spermine) with mediation of ornithine decarboxylase (EC 4.1.1.17). WAS leads to increased damage to lacrimal glands of rats on molecular level. Increased activity of iNOS may be the underlying mechanism of increased tissue damage during WAS. Introduction of metformin during WAS modeling led to decreased activity of NOS, including constitutive isoforms compared to WAS group and decreased nitrite concentration in lacrimal glands of rats compared to control group. Decreased iNOS activity is the result of metformin-dependent blockade of NF-κB activation [22]. Increase in SAR production is not an adverse effect, since its production during metformin introduction is lower compared to control group. Decreased iNOS activity impedes peroxynitrite formation during WAS correction by metformin and leads to decrease in LPO intensity. Increased activity of antioxidant enzymes, observed in this group, is the result stress-dependent Nrf-2 activation and subsequent upregulation of SOD and CAT genes transcription [23]. Quercetin, as the main active substance of corvitin, can downregulate expression of iNOS and upregulate Nrf-2 genes [24]. This explains our findings such as decreased iNOS and total NOS activity, increased activity of SOD and CAT, as well, as decreased intensity of LPO. SAR production in this group does not exceed the level of control animals. It is worth mentioning, that corvitin increased nitrite concentration in lacrimal glands of rats, which leads to suggestion of decreased peroxynitrite formation in the background of decreased iNOS activity. Both metformin and corvitin decrease activity of arginase during WAS modeling. This may be due to the decreased tissue damage in these groups, as proven by lowered MDA concentration. Lowered activity of cNOS in groups of experimental animals which received metformin and corvitin during WAS modeling requires further research and evaluation, since it may have adverse effects on metabolism of lacrimal glands of rats in longer perspective. Conclusions Increased activity of inducible NO-synthase during water avoidance stress is a possible reason of tissue damage in lacrimal glands of rats. Introduction of metformin or corvitin during water avoidance stress is an effective means for correction of tissue damage in lacrimal glands of rats due to their ability to lower increased inducible NO-synthase activity. References 1.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017; 15(3): 334-365. 2.Rouen PA, White ML. Dry Eye Disease: Prevalence, Assessment, and Management. Home Healthc Now. 2018; 36(2): 74-83. 3.Seen S, Tong L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018; 96(4): e412-e420. 4.Dogru M, Kojima T, Simsek C, Tsubota K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest Ophthalmol Vis Sci. 2018; 59(14): DES163-DES168. 5.Park B, Jo K, Lee TG, Hyun SW, Kim JS, Kim CS. Polydatin Inhibits NLRP3 Inflammasome in Dry Eye Disease by Attenuating Oxidative Stress and Inhibiting the NF-κB Pathway. Nutrients. 2019; 11(11): 2792. 6.Hyon JY, Yang HK, Han SB. Association between Dry Eye Disease and Psychological Stress among Paramedical Workers in Korea. Sci Rep. 2019; 9(1): 3783. 7.Cortes M, Esposito G, Sacco R, Gillet VB, Ianni A, Micera A. NGF and iNOS Changes in Tears from Video Display Terminal Workers. Curr Eye Res. 2018; 43(9): 1119-1125. 8.Kim J, Kim YS, Park SH. Metformin as a Treatment Strategy for Sjögren's Syndrome. Int J Mol Sci. 2021; 22(13): 7231. 9.Abengózar-Vela A, Schaumburg CS, Stern ME, Calonge M, Enríquez-de-Salamanca A, González-García MJ. Topical Quercetin and Resveratrol Protect the Ocular Surface in Experimental Dry Eye Disease. Ocul Immunol Inflamm. 2019;27(6):1023-1032. 10.Yamamoto K, Takao T, Nakayama J, Kiuchi H, Okuda H, Fukuhara S, Yoshioka I, Matsuoka Y, Miyagawa Y, Tsujimura A, Nonomura N. Water avoidance stress induces frequency through cyclooxygenase-2 expression: a bladder rat model. Int J Urol. 2012; 19(2): 155-62. 11.Talash VV, Kostenko VO. Effect of inhibitors of nuclear factor κB activation upon metabolism and hemocoagulation under modeled metabolic syndrome. Pharmacology and drug toxicology. 2015; 43(2): 83-9. (in Ukrainian) 12.Khmil’ DO, Kostenko VO. Effect of L-arginine and corvitin on oxidative-nitrosative stress in skin of rats exposed to excessive sodium nitrate. Fiziolohichnyi zhurnal. 2017; 63(6): 53-9. (in Ukrainian) 13.Akimov OYe, Kostenko VO. Functioning of nitric oxide cycle in gastric mucosa of rats under excessive combined intake of sodium nitrate and fluoride. Ukr. Biochem. J. 2016; 88(6):70-75.Crossref PubMed 14.Yelins’ka AM, Akimov OYe, Kostenko VO. Role of AP-1 transcriptional factor in development of oxidative and nitrosative stress in periodontal tissues during systemic inflammatory. Ukr.Biochem.J. 2019; 91(1): 80-5. 15.Kostenko VO, Tsebrzhins'kii OI. Production of superoxide anion radical and nitric oxide in renal tissues sutured with different surgical suture material. Fiziolohichnyi Zhurnal (Kiev, Ukraine : 1994). 2000 ;46(5):56-62. (in Ukraininan). 16.Brusov OS, Gerasimov AM, Panchenko LF. Effect of Natural Inhibitors of Radical Reactions on Adrenaline Autooxidation. Bulletin of Experimental Biology and Medicine. 1976; 1: 33-35. (In Russian). 17.Korolyuk MA, Ivanova LI, Mayorova IG. Method for determining catalase activity. Laboratory work. 1988; 1: 16-19. (In Russian). 18.Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998;11(10):1176-83. 19.Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018; 14: 618-625. 20.Jones AM, Vanhatalo A, Seals DR, Rossman MJ, Piknova B, Jonvik KL. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med Sci Sports Exerc. 2021 Feb 1;53(2):280-294. 21.Kapil V, Khambata RS, Jones DA, Rathod K, Primus C, Massimo G, Fukuto JM, Ahluwalia A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharmacol Rev. 2020 Jul;72(3):692-766. 22.Soydas T, Yaprak Sarac E, Cinar S, Dogan S, Solakoglu S, Tuncdemir M, Kanigur Sultuybek G. The protective effects of metformin in an in vitro model of aging 3T3 fibroblast under the high glucose conditions. J Physiol Biochem. 2018; 74(2): 273-281. 23.Batandier C, Poyot T, Marissal-Avry N, Couturier K, Canini F, Roussel AM, Hininger-Favier I. Acute emotional stress and high fat/high fructose diet modulate brain oxidative damage through NrF2 and uric acid in rats. Nutr Res. 2020; 79: 23-34. 24.Singh S, Singh DK, Meena A, Dubey V, Masood N, Luqman S. Rutin protects t butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine. 2019; 55: 92-104.
Information about authors and disclosure of information Corresponding author: Mykytenko A.O., e-mail: mykytenkoandrej18@gmail.com. Author contribution: Matsytska Ye.K.: Conception; Research; Visualization; Writing an initial project. Akimov O.Ye.: Research; Methodology; Formal analysis; Validation; Writing - reviewing and editing. Mykytenko A.O.: Conception; Overall responsibility; Validation; Writing - reviewing and editing. All authors analyzed the results and approved the final version of the manuscript. Disclaimer: Authors state that the opinions expressed in the submitted article are their own, and do not reflect official positions of their institution Financial support: there is no financial support for this work COI statement: Authors certify that we have no actual or potential conflict of interest (financial, personal, professional, or other interests) that I believe may be relevant to the subject matter or materials described and discussed in this manuscript. Abbreviations: WAS – water avoidance stress; ROS – reactive oxygen species; Nfr-2 – erythroid-2–related factor 2; SOD – superoxide dysmutases; CAT – catalase; NF-κB – nuclear factor kappa-light-chain-en hancer of activated B cells; iNOS – induc ible NO-synthase; MDA – malondialdehyde; NO2- – nitrites; ONOO- – peroxinitrites; LPO – lipid peroxidation; NO – nitric oxide; O2 – oxygen; O2•- – superoxide anion radical; CO2 – carbon dioxide; carbon trioxide (CO3•-); nitrate anion (NO3-).
|