J.ophthalmol.(Ukraine).2022;3:32-38.
|
http://doi.org/10.31288/oftalmolzh202233238 Received: 19.04.2021; Accepted: 04.05.2021; Published on-line 15.06.2022 Effect of experimental type 2 diabetes complicated by pyelonephritis on ltrastructural changes in the choroid, retina and nephrons Borisov S. O.1, Kostiev F. I. 1, Borisov O. V. 1, Dekhtiar Yu. M. 1, Vit V. V. 2, Molchaniuk N. I. 2, Mikheytseva I. M. 2, Kolomiichuk S. G. 2, Amaied Ahmed 2 1 Odesa National Medical University; Odesa (Ukraine) 2 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE: Borisov SO, Kostiev FI, Borisov OV, Dekhtiar YuM, Vit VV, Molchaniuk NI, Mikheytseva IM, Kolomiichuk SG, Amaied Ahmed. Effect of experimental type 2 diabetes complicated by pyelonephritis on ultrastructural changes in the choroid, retina and nephrons. J.ophthalmol.(Ukraine).2022;3:32-8. http://doi.org/10.31288/oftalmolzh202233238
Background: The clinical and morphological picture of diabetic microangiopathy is rather specific. Diabetic retinal ischemia can lead to irreversible damage to retinal neural elements and choroidal capillaries. Diabetic nephropathy can lead to progressive renal dysfunction and chronic renal failure. Choroidal and retinal capillaries are structurally and functionally similar to those of the intestinal mucosa and renal tissue. Purpose: To assess vascular ultrastructural changes in the choroid, retina, and renal glomerular and tubular system in a rat model of pyelonephritis in the presence of type 2 diabetes. Material and Methods: Samples were obtained from 95 Wistar rats divided into three groups: group 1 (or control group) of 30 intact animals; group 2 of 15 animals with type 2 diabetes induced by intraperitoneal streptozotocin 15.0 mg/kg for 5 consecutive days; and group 3 of 50 animals with acute pyelonephritis in the presence of type 2 diabetes (streptozotocin 35.0 mg/kg on 2 days spaced by a week). Acute pyelonephritis was induced by Escherichia coli administration (107 CFU/kg) rectally. The ultrastructure of rat choroidal, retinal and renal glomerular-and-tubular vessels was examined with electron microscopy (PEM-100-01 electron microscope). Results: In rats with induced type 2 diabetes, the most significant ocular vascular changes and renal vascular changes were found in endothelial cells. These changes included findings of vacuolar degeneration in some epithelial cells, basal membrane thickening and focal necrosis of individual epithelial cells. Vessel lumens appeared focally narrowed or expanded, with red blood cells forming clumps or sludge in lumens. Some capillaries were obliterated. These changes obviously caused secondary changes in the surrounding structures. Common ocular changes included focal destruction in retinal pigment epithelium cells, destruction of retinal photoreceptor inner segments and choroidal stromal edema. Common renal changes included destruction of the podocytes of the glomerular capillary network and homogenization of the basal membrane. Vascular ultrastructural changes in the renal glomerular system were more marked in rats with experimental type 2 diabetes and pyelonephritis than in those with type 2 diabetes only. Conclusion: Electron microscopy micrographs demonstrated the ultrastructural changes in the retinal and uveal vascular systems which were of a similar type to those in the renal glomerular-and-tubular system in rats with experimental pyelonephritis in the presence of type 2 diabetes. Keywords: ultrastructure, pyelonephritis, type 2 diabetes, choroid, retina, renal glomeruli and tubules
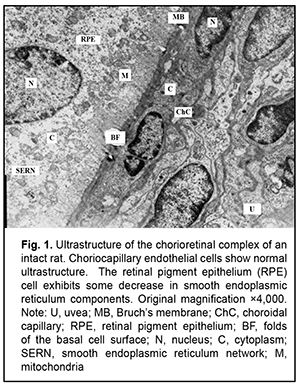
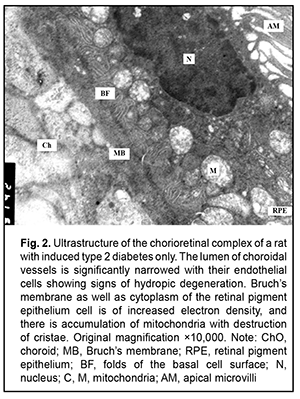
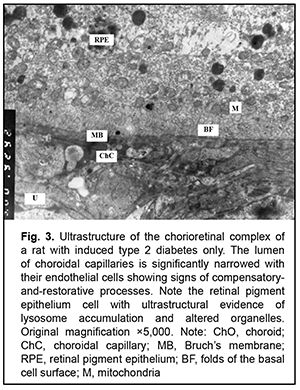
Introduction The clinical and morphological picture of diabetic microangiopathy is rather specific. Of all the vascular complications, diabetic nephropathy and retinopathy are worthy of the most attention [1]. Diabetic nephropathy is one of the most severe complications of type 2 diabetes, because it can lead to progressive renal dysfunction and chronic renal failure, with patients having an irreversible and even pessimistic prognosis [1, 2]. Another organ vulnerable to hyperglycemia is the eye, with diabetic retinopathy being the most dangerous diabetic ocular complication leading to impaired vision and blindness [3-6]. Capillary microocclusion and hyperpermeability contribute to this pathological process. Under these circumstances, blood retinal barrier breakdown develops, with subsequent growth of vessels and pathological connective tissue leading to massive hemorrhage and retinal detachment [7]. Metabolic alterations and hemodynamic factors leading to ischemia are preceded by changes at the cellular level that affect microcirculations and angiogenesis [4, 8-10]. The retina is the most commonly affected. Because the retina is metabolically highly active, ischemia may lead to irreversible damage to retinal neural elements and choroidal capillaries. Neurodegeneration is accompanied by neuronal apoptosis, glial dysfunction and an increased vascular permeability [3, 10]. The percentage of choroid with focal choriocapillaris degeneration in diabetic choroids had been found to be more than 4-fold greater than that in nondiabetic choroids [11]. Polymorphonuclear leukocytes contribute to vaso-occlusive processes and endothelial cell injury in the diabetic choroid [12]. The presence of leukocytes in the lumen indicates uveal inflammation [3]. It is noteworthy that choroidal and retinal capillaries are structurally and functionally similar to those of the intestinal mucosa and renal tissue [13, 14]. Consequently, the purpose of this study was to assess vascular ultrastructural changes in the choroid, retina, and renal glomerular and tubular system in a rat model of pyelonephritis in the presence of type 2 diabetes. Material and Methods Samples were obtained from 95 Wistar rats divided into three groups: group 1 (or control group) of 30 intact animals; group 2 of 15 animals with type 2 diabetes induced by intraperitoneal streptozotocin 15.0 mg/kg for 5 consecutive days; and group 3 of 50 animals with acute pyelonephritis in the presence of type 2 diabetes (streptozotocin 35.0 mg/kg on 2 days spaced by a week). Acute pyelonephritis was induced by Escherichia coli administration (107 CFU/kg) rectally. All animal experiments were performed in compliance with the Law of Ukraine on Protection of Animals from Cruel Treatment No. 3447-IV and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), and approved by a local Bioethics Committee of the Filatov Institute. For electron microscopy, tissue samples were fixed in 2.5% glutaraldechyde in phosphate buffer at pH 7.4 and post-fixed in 1% osmic axid in phosphate buffer at pH 7.4. Sections were dehydrated with ascending grades of alcohol and embedded in Epon-araldite mixture. Ultrathin sections of rat choroidal, retinal and renal glomerular-and-tubular tissue were cut, stained with Reynolds’ lead citrate [15], and examined in a PEM-100-01 electron microscope [15]. Results Ultrastructural features of choroidal and retinal vessels Choroidal tissue samples of rats with induced type 2 diabetes appeared edematous. The lumen of most vessels and capillaries was narrowed due to large swollen endothelial cells. At other sites, the lumens of normal diameter were filled with red blood cells. The ultrastructure of endothelial cells of the choriocapillaris was heterogeneous: some cells showed normal structure, whereas others showed signs of hydropic degeneration. In addition, there were sites with signs of compensatory-and-restorative processes, with increased amount of cytoplasmic organelles and hypertrophic nucleus with diffuse heterochromatin. It is noteworthy that fenestrations were well seen at the thinned cytoplasmic sites of endothelial cells, and solitary microvilli were seen at the luminal surface of these cells. However, lumens of vessels and capillaries contained structures of increased electron density which were visualized at the Bruch's membrane. This indicated blood retinal barrier breakdown, which facilitated diffusion of substances via RPE cell membranes. This in turn indicated altered permeability of cytoplasmic membranes of choriocapillary endothelial cells and RPE cells. We observed heterogeneous changes in RPE cells. Focally, there were cells with normal structure, whereas others exhibited hyaloplasm and karyoplasm of increased electron density and some altered mitochondria. In addition, there were cells with signs of compensatory-and-restorative processes. Each of the latter cells had two nuclei with dispersed chromatin, high number of mitochondria and focal ultrastructural evidence of altered smooth endoplasmic reticulum components and lysosome accumulation. It is noteworthy that the basal surfaces of RPE cells showed the absence of or poorly developed folds at some sites, indicating impaired transport processes between the chorioretinal complex and RPE cells (Figs. 1, 2 and 3).
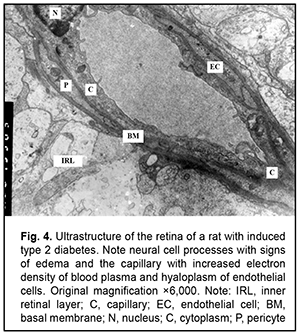
The severity of changes in retinal photoreceptor cells depended on the severity of changes in RPE cells. Most capillary endothelial cells showed normal ultrastructure. Endothelial cells in individual capillaries showed clear hyaloplasm and a reduced amount of organelles. Endothelial cell basal membrane appeared thickened, whereas pericytes had an almost normal ultrastructure (Fig. 4).
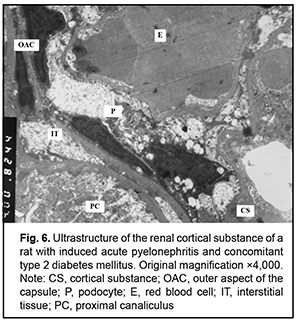
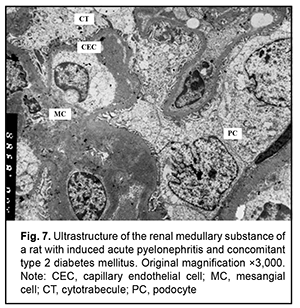
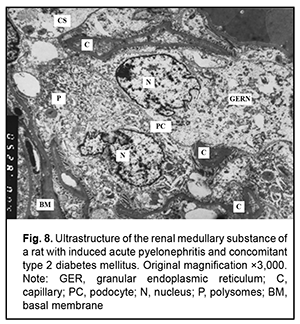
It is noteworthy that both mild hydropic changes and severe hydropic changes (i.e., hydropic degeneration) were observed in retinal endothelial cells of animals with acute pyelonephritis in the presence of type 2 diabetes. Ultrastructural features of renal tissues in acute pyelonephritis complicated by type 2 diabetes Normally, the renal glomerular system has a well-developed capillary network. Endothelial cell bodies were found bridging the lumen in some of capillaries. Cells had large nuclei with nuclear membrane invaginations and folds. The karyoplasm as well as cytoplasm was electron light. In the nucleus, chromatin margination was seen. Diffuse chromatin was seen in the central region. The endothelial cell cytoplasm contained diffusely distributed individual mitochondria, Golgi cisterns, polysomes and small pinocytosis vesicles. Individual red blood cells were seen in the lumen. Fenestrations were well seen at the thinned cytoplasmic sites of endothelial cells. Type 2 diabetes has caused significant ultrastructural changes in the renal tissue of rats with acute pyelonephritis compared to controls and rats with acute pyelonephritis only [16]. It is noteworthy that the examined renal cortical structures containing the glomerular substance (Figs. 5 and 6), appeared less preserved than renal medulla structures (Figs. 7 and 8).
The ultrastructure of the renal cortical substance in rats with induced acute pyelonephritis and concomitant type 2 diabetes (Fig. 5) shows structurally deformed and narrowed capillaries with altered capillary endothelial cells, red blood cell sludge and no cytopodia at an extensive site. The Bowman’s space was seen bridged by hypertrophied podocytes. Renal cortical substance of the rats of this group exhibited destruction of the podocytes of the inner and outer leaflets, destruction of glomerular capillary epithelial cells and sludge of capillary red blood cells. In addition, there was no Bowman’s space and destruction of organelles of proximal tubule epithelial cells and interstitial tissue cells was seen (Fig. 6). Ultrastructure of the medullary substance of rats with induced acute pyelonephritis and concomitant type 2 diabetes showed hydropic changes and destruction of organelles in cytotrabecules and endothelial cells of nephron capillary networks with focal cytopodial destruction (Fig. 7). It is noteworthy that reduced capillary filling and hypertrophied podocytes under destruction were characteristic for the medullary substance of the rats of this group (Fig. 8). Therefore, summing up, rats with induced acute pyelonephritis and concomitant type 2 diabetes exhibited renal glomerular capillaries with endothelial cells of increased electron density, a large round nucleus and swollen mitochondria. Glomerular capillaries appeared structurally deformed, nodular and focally significantly narrowed or collapsed. The capillary lumens were filled with red blood cells. Unlike the glomerular capillaries of intact animals, those of rats with induced acute pyelonephritis and concomitant type 2 diabetes contained endothelial cells of increased electron density, a large round nucleus and swollen mitochondria. Some capillary endothelial cells showed signs of hydropic degeneration, with red blood cells, either individual or forming clumps or sludge in capillary lumens. In addition, fenestrations in these endothelial cells were poorly determined. Other capillaries were of normal structure or their endothelial cells had focal loss of organelles with signs of cytoplasmic matrix swelling and cell hypertrophy (Fig. 5). A portion of capillaries appeared structurally deformed and narrowed. In many capillaries, endothelial cells were either absent or necrosed. A portion of these cells had round nuclei with nuclear membrane invaginations and folds, and chromatin appeared almost destroyed (Figs 5 and 6). It is noteworthy that structural and functional alterations of renal vascular endothelial cells caused significant changes primarily in the surrounding podocytes. We noted also homogenization of the basal membrane and extensive mesangial cell growth. Podocytes of the outer leaflet had a cytoplasm of high electron density, others had a hypertrophied nucleus and were irregularly shaped, and a portion of podocytes was under destruction. Many podocytes showed increased size and their cytoplasm appeared light and swollen. Cytoplasmic organelles of podocytes and podocyte pedicles showed various degrees of destruction. Some of these cells appeared empty (Figs 5 and 6). Discussion In hyperglycemia, pathological changes (e.g., diabetic nephropathy [2, 16, 17] and retinopathy) develop in many organs and body systems, resulting in complex functional alterations in these organs [3-6]. In the current study, the ultrastructural changes in the retinal and uveal vascular systems were of a similar type to those in the renal glomerular-and-tubular system in rats with type 2 diabetes. We found that induced acute pyelonephritis and concomitant type 2 diabetes caused more severe ultrastructural changes in the kidneys than in the retina, with the most significant ocular vascular changes and renal vascular changes found in endothelial cells. These changes included findings of vacuolar degeneration in some endothelial cells and capillary basal membrane thickening. There was focal necrosis of individual epithelial cells. Vessel lumens appeared focally narrowed or expanded, with red blood cells forming clumps or sludge in lumens. Some capillaries were obliterated. These changes obviously caused secondary changes in the surrounding structures. Common ocular changes included focal destruction in RPE cells, destruction of retinal photoreceptor inner segments and choroidal stromal edema. Common renal changes included destruction of the podocytes of the glomerular capillary network and homogenization of basal membrane, with the cortical portion of the kidneys being more sensitive to the pathogenic effect of streptozotocin than the kidney medullary substance, which results in persistent hyperglycemia. Thus, there were structural alterations in renal cortical parenchyma with morphological manifestations of early mesangial cell apoptosis, and the changes in the renal medullary layer were consistent with acute infectious inflammation in rats with induced pyelonephritis in the presence of type 2 diabetes [18]. Conclusion In the current study, electron microscopy micrographs demonstrated the ultrastructural changes in the retinal and uveal vascular systems which were of a similar type to those in the renal glomerular-and-tubular system in rats with experimental pyelonephritis in the presence of type 2 diabetes.
References 1.Ighodaro OM, Adeosun АМ. Vascular complications in diabetes mellitus. Glob J Endocrinol Metab. 2017;1(2):1–3. 2.Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005 Jan;28(1):164-76. 3.Maltsev EV, Zborovska OV, Dorokhova OE. [Fundamental aspects of the development and treatment of diabetic retinopathy]. Odesa: Astroprint; 2018. Russian. 4.Maltsev EV, Zborovska OV, Dorokhova ОE. [State of the retina and choroid in the rabbit with dithizone-induced diabetes. Report 1. Structural changes]. Oftalmol Zh. 2011;6: Russian. 5.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. 6.Keen H, Lee ET, Russell D, et al. The appearance of retinopathy and progression to proliferative retinopathy: The WHO multinational study of vascular disease in diabetes. Diabetologia. 2001 Sep;44 Suppl 2:S22-30. 7.Oganezova ZhG, Egorov EA. [The use of angioprotectors in the treatment of diabetic angiopathy: focus on calcium dobesilate]. RMJ Klinicheskaia oftalmologiia. 2015;4:201–4. Russian. 8.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615-25. 9.Bhavsar AR, Emerson GG, Emerson MV, Browning DJ. Diabetic Retinopathy. Epidemiology of Diabetic Retinopathy. In: Browning DJ, editor. Diabetic Retinopathy: Evidence-Based Management. New York: Springer; 2010. 10.Gusev YuA, Kapkova SG, Varkentina IV, Tret'yak EB. [Calcium dobesilate in the treatment of diabetic retinopathy: the most important thing is not to lose time]. RMJ Klinicheskaia oftalmologiia. 2016;1:50–4. Russian. 11.Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998 May;116(5):589-97. 12.Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes to capillary dropout in the human diabetic choroids. Am J Pathol. 1997 Sep;151(3):707-14. 13.Vit VV. [The structure of the human visual system]. Odesa: Astroprint; 2003. Russian. 14.Vit VV. [Pathology of the eye, ocular adnexa and orbit]. Odesa: Astroprint; 2019. Russian. 15.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. 1963 Apr;17(1):208-12. doi: 10.1083/jcb.17.1.208. 16.Borysov SO, Kostyev FI, Borysov OV, Molchanyuk NI. [Electron microscopic diagnostics of apoptosis processes under simulation conditions in the experiment of acute pyelonephritis and concomitant diabetes mellitus type I and II]. Reports of Morphology. 2018;24(1):39-46. 17.Hrytsiuk NI. [Changes in tortuous canaliculi after NADP injection in rats with streptozotocin-induced diabetes]. Mizhnarodnyi endokrynologichnyi zhurnal. 2017;13(8):624-9. Ukrainian. 18.Borysov SO, Kostiev FI, Borysov OV, Artiomov OV. [Comparison of renal morphological changes in rat models of pyelonephritis and concomitant type 1 or 2 diabetes mellitus]. Urologiia. 2019. 23(3):205-10. Disclosures Corresponding Author: Kolomiichuk Serhii, fiatovbiochem@ukr.net, Author contribution: Borisov S.O.: preparation of the initial project, modeling of acute pyelonephritis on the background of type II diabetes, analysis of results, writing; Kostev F.I.: concept, design, analysis, reviewing and editing; Borisov O.V.: concept, design, reviewing and editing; Dekhtyar Yu.M.: concept, reviewing and editing; Vit V.V.: concept, reviewing and editing; Molchanyuk N.I.: data analysis and interpretation (ultrastructure of the vessels of the choroid, retina, tubules and glomeruli of the rat kidneys); Mikheytseva I.M.: concept, design, reviewing and editing; Kolomiychuk S.G.: modeling of type II diabetes, writing; Ahmed Amaied: modeling of type II diabetes, dat analysis, writing. All authors analyzed the results and approved the final version of the manuscript. Conflict of Interest. The authors state that there is no conflict of interest that could affect their opinion on the subject or materials described and discussed in this manuscript. Sources of support. None The article is part of research on "The role of cellular and tissue metabolism in the diagnosis, clinical course and treatment of diseases of the kidneys, urinary tract and genitals" №State Registration 0121U108881, 2021-2025; "Clinical and experimental study in determining the pathogenetic role of metabolic disorders of the anterior and posterior part of the eye in type II diabetes with myopia" № state registration 0119U101071, 2019-2021. Disclaimer: The opinions presented in this article are solely those of the author and not the official position of the institutions.
|