J.ophthalmol.(Ukraine).2022;3:24-31.
|
http://doi.org/10.31288/oftalmolzh202232431 Received 09.02.2022 Accepted 06.06.2022 Published on-line 15.06.2022 Use of Vasavital® in patients with diabetic retinopathy Khramenko N. I. 1, Umanets M. M. 1, Medvedovska N. V. 2, Levytska G. V. 1, Rozanova Z. A. 1 1 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the N AMS of Ukraine"; Odesa (Ukraine) 2 National Academy of Medical Science of Ukraine; Kyiv (Ukraine) TO CITE THIS ARTICLE:Khramenko NI, Umanets MM, Medvedovska NV, Levytska GV, Rozanova ZA. Use of Vasavital® in patients with diabetic retinopathy. J.ophthalmol.(Ukraine).2022;3:24-31. http://doi.org/10.31288/oftalmolzh202232431
Background: Diabetic retinopathy (DR) is a major cause of visual impairment or blindness among working-age adults worldwide. For years, researchers around the world have been trying to develop new effective pharmaceutical methods of treatment for preclinical and early DR. Purpose: To examine the effect of a one-month course of Vasavital on the function of the visual system and ocular hemodynamics (using ophthalmic rheography) in patients with non-proliferative and proliferative diabetic retinopathy (NPDR and PPDR, respectively). Material and Methods: Forty-seven type 2 diabetes patients with DR and moderate glycemic control were divided into those with NPDR (group 1 of 15 patients; 30 eyes) and those with PPDR (group 2 of 17 patients; 34 eyes). The control group was composed of 15 volunteers (30 eyes) of similar age having no systemic or eye disease. Patients received a one-month course of Vasavital-only therapy at a dose of one capsule twice a day as an outpatient treatment. They received visual acuity assessment, intraocular pressure measurement, ophthalmoscopy, biomicroscopy, perimetry, systemic blood pressure and pulse measurement, optical coherence tomography and fluorescein angiography, and ocular hemodynamics was assessed by ophthalmic rheography. Eleven patients (22 eyes) with NPDR and ten patients (20 eyes) with PPDR underwent electrophysiological studies of electrically evoked phosphene threshold (EEPT) and critical frequency of phosphene disappearance (CFPD), before and after a course of Vasavital treatment. Results: Patients reported that a one-month course of Vasavital was well-tolerated, with no new complaints. In addition, no side effects were observed. After treatment, the function of the photopic afferent system as assessed by light sensitivity at minutes 0 to 7 of adaptation improved by 33.3%-40% in patients with NPDR and by 27.2%-33.3% in patients with PPDR. In addition, there was a decrease in EEPT by 18% and 7.7%, respectively, and an increase in CFPD by 28.2% and 24.7%, respectively, for patients in groups 1 and 2. Moreover, ocular pulse blood filling improved by 27.7% in patients with NPDR and by 17.3% in patients with PPDR, and vascular tone in large-caliber vessels decreased by 8% in the former patients. Conclusion: A one-month Vasavital course administered to patients with DR had a positive effect on the visual system function and ocular circulation parameters, which provides grounds for the use of the Ginkgo biloba-based preparation as a monotherapy or as part of a combined treatment for initial functional changes in the visual system in DR. Keywords: diabetic retinopathy, Vasavital, visual system function, ophthalmic rheography
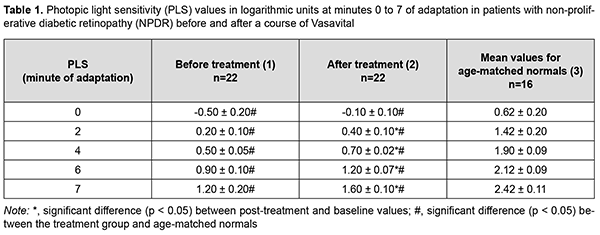
Introduction The prevalence of diabetes had been steadily increasing for decades, and, globally, an estimated 422 million adults were living with diabetes in 2014 [1]. The number of people living with diabetes is expected to increase to 578 million in 2030 and 700 million in 2045 [2]. Therefore, diabetes prevention, improving the opportunities for early diagnosis, sustaining the lifestyle changes needed to reduce risk, and presenting grounds for pharmaceutical interventions aimed at preventing or delaying the progression or reversing the complications of diabetes are essential [1, 2]. Diabetic retinopathy (DR) is a major cause of visual impairment or blindness among working-age adults worldwide [3, 4]. A study has provided the first global estimate of DR and, more important, the two sight-threatening end points (proliferative DR and diabetic macular edema), and reported that 35% of people with diabetes had some form of DR, and that 10% were affected by vision-threatening stages of DR [5]. The development of DR is associated with sustained metabolic disorders caused by hyperglycemia and increased levels of inflammatory cytokines (like vascular endothelial growth factor (VEGF) and tumor necrosis factor (TNF)-α and β) in the blood. Systemic inflammation caused by these metabolic alternations leads to hemodynamic changes, blood-retinal barrier (BRB) damage, the leakage of retinal microvessels and edema, a gradual thickening of the retinal vascular basement membrane, a loss of pericytes, and the development of microaneurysms. These structural and functional alterations are accompanied by microvascular occlusion, neovascularization and neurodegeneration. Oxidative stress is thought to be one of the crucial factors in the pathogenesis of DR. Abnormal metabolism induced by hyperglycemia can result in the overproduction of free radicals such as hydroxyl and superoxide radicals, which are known as reactive oxygen species (ROS). The imbalance between ROS production and the antioxidant defense system activates several oxidative stress-related mechanisms that promote the pathogenesis of DR [6, 7]. Bungau and colleagues [8] searched PubMed and Web of Science databases for original studies investigating the benefits of different carotenoids and polyphenols in age-related ophthalmic diseases (including DR), and found that some polyphenols several polyphenols (such as anthocyanins, Ginkgo biloba, quercetin, and resveratrol) and carotenoids (such as lutein, zeaxanthin, and mezoxanthin) have shown significant preventive and therapeutic benefits against the aforementioned conditions. The beneficial effects of polyphenols include: scavenging free radicals, reducing the formation of glycation end products, inhibiting aldose reductase, ameliorating inflammation, and improving ocular blood flow and signal transduction [9]. While looking for novel effective and safe agents for the treatment of mild DR, we considered Vasavital®, a preparation composed of Ginkgo biloba leaf extract, a set of vitamins and bee pollen pellet. Each Vasavital® capsule contains 40 mg of standardized extract of Ginkgo biloba leaves, 60 mg of bee pollen pellet, and a set of vitamins (thiamine, 1 mg; riboflavin, 1 mg; pyridoxine, 1 mg; ascorbic acid, 30 mg; rutin, 20 mg; and nicotinic acid, 17 mg). Ginkgo biloba extract (GBE) contains a group of ginkgo flavone glycosides and terpenoids. It is well known that GBE has an antioxidant action as a free radical scavenger, and an anti-inflammatory effect suppressing the production of active oxygen and nitrogen species. It inhibits the increase in the products of the oxidative decomposition of low-density lipoprotein (LDL), reduces the cell death in various types of neuropathy, and prevents the oxidative damage to mitochondria, suggesting that its beneficial effects on neurodegenerative diseases are related to prevention of chronic oxidative damage [10]. Our preliminary experience with the use of Vasavital® has demonstrated its beneficial effect on visual function and ocular hemodynamics in dry age-related macular degeneration and ischemic optic neuropathy [11]. Because Vasavital® had various pharmaceutical effects, and given our previous positive clinical experience of its use against the eye disease with basic ischemic process, we decided to assess the therapeutic effect of a course of Vasavital® in patients with non-proliferative and proliferative DR. The purpose of the study was to examine the effect of a one-month course of Vasavital on the function of the visual system and regional hemodynamics (using ophthalmic rheography) in patients with non-proliferative and proliferative diabetic retinopathy. Material and Methods Forty-seven patients with DR underwent examination and received medical advice at the Department of Vitreoretinal Surgery and Laboratory for Functional Examination of the Visual System of the Filatov Institute. These patients were divided into those with non-proliferative DR (NPDR; group 1 of 15 patients; 30 eyes) and those with pre-proliferative DR (PPDR; group 2 of 17 patients; 34 eyes). Both eyes of all patients with NPDR had ischemic hemorrhagic retinopathy. In addition, of the eyes with NPDR, 4 had maculopathy, and 10, diffuse macular edema. Of the eyes with PPDR, 6 had ischemic hemorrhagic retinopathy, and 28, exudative hemorrhagic retinopathy. In addition, of the eyes with PPDR, 5 had focal macular edema, 27, diffuse macular edema, and 2, complicated macular edema as per the 2009 Pasyechnikova and Naumenko’s classification [12]. The mean patient age was 66.8 years ± 6.8 (standard deviation). All patients had type 2 diabetes with moderate glycemic control. The mean diabetes duration was 10.6 years ± 5.6 (standard deviation) and blood glucose level ranged from 7.7 mmol/L to 9.9 mmol/L. Systolic blood pressure ranged from 130 to 150 mm Hg and diastolic blood pressure from 80 to 90 mm Hg. The control group was composed of 15 volunteers (30 eyes) of similar age having no systemic or eye disease. Patients received a one-month course of Vasavital-only therapy at a dose of one capsule twice a day as an outpatient treatment. They received visual acuity assessment, intraocular pressure measurement, ophthalmoscopy, biomicroscopy, perimetry, systemic blood pressure and pulse measurement, optical coherence tomography and fluorescein angiography, and ocular hemodynamics was assessed by ophthalmic rheography. Eleven patients (22 eyes) with NPDR and ten patients (20 eyes) with PPDR underwent electrophysiological studies, including studies of electrical sensitivity of the visual system (electrically evoked phosphene threshold, EEPT), lability of the optic nerve (critical frequency of phosphene disappearance, CFPD), and light sensitivity of the photopic afferent system (by adaptometry), before and after a course of Vasavital treatment. Patients reported their complaints prior to and after a one-month course of Vasavital-only therapy. Electrical sensitivity of the optic nerve (EEPT) and lability of the optic nerve e (CFPD) were assessed using electric phosphene stimulation with a KNSO-2 apparatus (FOSFEN, Odesa, Ukraine). EEPT was assessed after light adaptation at 1 minute of darkness adaptation. A tip electrode connected both to the current generator and the plate electrode was applied onto the closed eyelid. A patient was exposed to single 10-ms current pulses of increasing current strength until phosphene perception was achieved, and, in this way, an EEPT value was obtained. Thereafter, current strength was increased to 300% or 150% of phosphene threshold, the patient was exposed to current pulses of increasing frequency (10 Hz to 60 Hz) until flickering phosphenes disappeared, and the frequencies obtained in this way were recorded as CFPD3 and CFPD1.5, respectively [13]. The normal values determined at the Laboratory for Functional Examination of the Visual System and stated in the Ponomarchuk’s monograph [13] were used as normal EEPT, CFPD3 and CFPD1.5 values in this paper. Patients underwent ophthalmic rheography (ORG) with Reocom (KHAI-Medika, Kharkiv, Ukraine), the computerized rheography apparatus, with its operation based on the principle of impedancemetry. ORG included measurements of ocular pulse blood filling (OPBF, expressed as RQ, ‰ rheographic coefficient) and vascular tone (expressed as alpha/T percentage index). Vascular tones in large-caliber vessels and small-caliber vessels were determined using high-frequency and low-frequency characteristics of the differential ROG curve. Volumetric ocular blood flow velocity was determined for the rising portion of ROG curve and assessed in Ohm per second. Light sensitivity was assessed with a semiautomatic adaptometer during a 10-minute session that included 3 minutes of light adaptation (luminance, 1200 cd/m2) and 7 minutes of dark adaptation (with luminance adjusted from 2х10 – 8 cd/m2 до 7.0 cd/m2), and measurements were performed at baseline and at minutes 2, 4, 6 and 7 of the dark adaptation period. The study was conducted in accordance with applicable local laws and the principles stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine. Written informed consent was obtained from all participants of the study. Statistical analyses were conducted using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. Data are presented as mean (M), error of mean (m), and standard deviation (SD). A pairwise comparison was performed by using the paired Wilcoxon signed-rank test. Results Patients reported that a one-month course of Vasavital was well-tolerated, with no new complaints. In addition, no side effects were observed. Prior to this treatment, best-corrected visual acuity (BCVA) in patients of group 1 and group 2 was 0.64 ± 0.45 and 0.53 ± 0.43, respectively. A one-month course of Vasavital resulted in no substantial change in visual acuity. Photopic light sensitivity was assessed through the determination of light sensitivity thresholds. Low-threshold photic stimulation (or the absolute light threshold) is the weakest photic stimulation that causes light perception. Absolute light threshold or minimum threshold is the minimum light stimulation causing light perception. The reciprocal of the absolute light threshold characterizes the light sensitivity. Light perception is preformed at the retinal photosensor level. Visual pigment molecules in photoreceptor outer segments absorb light quanta and undergo decomposition to ensure a sequence of photochemical, electrical, ionic and enzymatic processes. When retinal architectonics is impaired and/or ischemic process or metabolic starvation occurs, light sensitivity, one of the oldest and major functions of the visual system, becomes the most sensitive to changes in tissue blood supply. After a course of Vasavital treatment, photopic light sensitivity of patients with NPDR significantly improved. At minute 2 of adaptation, there was a twofold increase in photopic light sensitivity threshold compared to baseline values, and this increase was statistically significant (p < 0.05; Table 1). In addition, at minutes 4, 6 and 7 of adaptation, photopic light sensitivity threshold increased 40% (p < 0.05), 33% (p < 0.05), and 33% (p < 0.05), respectively, compared to baseline measurements (Table 1).
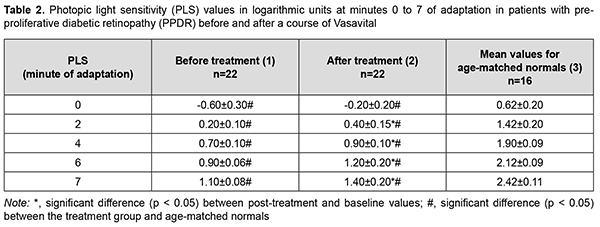
Pretreatment photopic light sensitivity threshold values were two-fold to 7.1-fold decreased compared to controls (p < 0.05; Table 1). After treatment, photopic light sensitivity threshold values for patients with NPDR significantly improved, but were 1.5-fold to 3.6-fold decreased compared to controls (p < 0.05; Table 1). After a course of Vasavital treatment, photopic light sensitivity of patients with PPDR increased twofold (p < 0.05) at minute 2, 28.5% (p < 0.05) at minute 4, 33.3% (p < 0.05) at minute 6, and 27.2% (p < 0.05) at minute 7 of adaptation (Table 2). Pretreatment photopic light sensitivity threshold values for patients with NPDR were 2.2-fold to 7.1-fold decreased compared to controls (p < 0.05; Table 2). After treatment, photopic light sensitivity threshold values for patients with PPDR significantly improved, but were 1.7-fold to 3.6-fold decreased compared to controls (p < 0.05; Table 2).
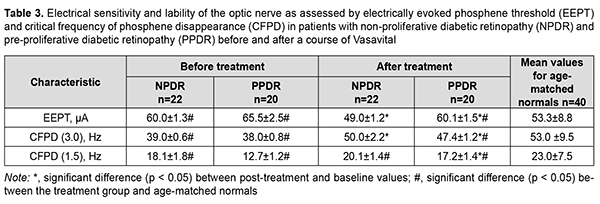
Prior to treatment, electrically evoked phosphene threshold (EEPT) was 60.0 ± 1.3 µA for patients of group 1 and 65.5 ± 2.5 µA for patients of group 2, which was 12.6% (p < 0.05) and 22.8% (p < 0.05), higher, compared to controls, and this difference was significant (Table 3). After treatment, EEPT decreased by 18% (p < 0.05) compared to baseline and became normal for patients of group 1, and decreased by 7.7%% (p < 0.05) compared to baseline but was 13% (p < 0.05), higher compared to controls for patients of group 2 (Table 3). These post-treatment changes in EEPT in patients of both groups reflected an improved function of internal retinal layers and visual pathway. In addition, after this treatment, critical frequency of phosphene disappearance (CFPD3) for patients in group 1 and group 2 increased by 11 Hz (28.2%; p < 0.05) and 9.4 Hz (24.7%; p < 0.05), respectively (Table 3). Moreover, after this treatment, CFPD3 for patients in group 1 normalized, and for patients in group 2, was 10% lower (p < 0.05) than in controls (Table 3).
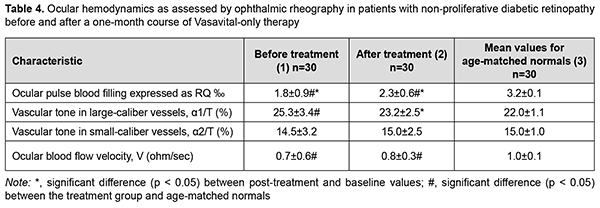
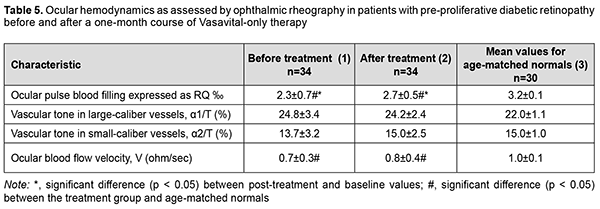
Critical frequency of phosphene disappearance reflects the function of the axial bundle of the optic nerve. The determination of CFPD with the current strength increased to 300% 150% of phosphene threshold (i.e., CFPD1.5) is considered a more sensitive method [13]. After treatment, CFPD1.5 for patients in group 1 did not change substantially, and for patients in group 2 increased by 4.5 Hz (35%; p < 0.05) (Table 3), indicating that the function of the axial bundle of the optic nerve in the former patients was rather good, and the conductivity of the axial bundle of the optic nerve in the latter patients improved. However, after treatment, CFPD1.5 for patients in group 1 and group 2 was 13% lower (p < 0.05) and 25% lower (p < 0.05), respectively, compared to controls (Table 3). Therefore, our electrophysiological studies demonstrated that, after treatment, there was a decrease in electrically evoked phosphene threshold by 18% and 7.7%, respectively, and an increase in CFPD by 28.2% and 24.7%, respectively, for patients in groups 1 and 2, and an increase in CFPD1.5% by 35% for patients in group 2. This reflects an improved function of the inner retina and the axial bundle of the optic nerve in both groups of patients with treatment. All patients underwent ophthalmic rheography study before and after treatment to determine changes in their ocular hemodynamics. In patients of group 1, OPBF (expressed as RQ, ‰ rheographic coefficient) was 1.8 ± 0.9‰ at baseline (which was 43.8% lower than in age-matched normals, p < 0.05) (Table 4), and increased to 2.3 ± 0.6 ‰ (by 27.7%, p < 0.05) and was still lower than in age-matched normals after a one-month course of Vasavital (Table 4).
In patients of group 2, OPBF (expressed as RQ, ‰ rheographic coefficient) was 2.3 ± 0.7‰ at baseline (which was 28.1% lower than in age-matched normals, p < 0.05), and increased by 17.3% (p < 0.05) after treatment (Table 5).
Vascular tone in large-caliber vessels (expressed as alpha1/T percentage index) decreased by 8% (p < 0.05) in patients in group 1, and did not change substantially in patients in group 2, after a one-month course of Vasavital. Volumetric blood flow velocity tended to increase in all groups after a course of Vasavital monotherapy. Therefore, a course of treatment with Vasavital resulted in improvement in visual system function and ocular hemodynamics. The function of the photopic afferent system as assessed by light sensitivity at minutes 0 to 7 improved by 33.3%-40% in patients with NPDR and by 27.2%-33.3% patients with PPDR. In addition, in the former and latter patients, the function of the inner retina and visual pathway as assessed by EEPT improved by 18% and 7.7%, respectively, and axial bundle conductivity as assessed by CFPD improved by 28.2% and 24.7%, respectively. Moreover, ocular pulse blood filling improved by 27.7% in patients with NPDR and by 17.3% patients with PPDR, and vascular tone in large-caliber vessels (expressed as alpha1/T percentage index) decreased by 8% in the former patients. Discussion Various plant extracts and their therapeutic effects have gained increased attention. GBE is an extract of leaves of Ginkgo biloba, an ancient survivor. Commercially available GBE consists of 60 bioactive compounds. In the most widely used EGb 761 extract, the 2 major component groups are flavonoids and terpenoids. Flavonoids constitute 24% to 27% of the extract, examples of which are biflavones, catechin derivatives, flavonol glycosides, and 7% proanthocyanidins. Terpenoids make up 5% to 7% of the extract. The extract also contains alkylphenols (ginkgolic acids) and organic acids [14,15]. Under normal physiological conditions, GBE increases endothelial nitric oxide (NO) levels, leading to vasodilation and an increase in blood flow. Additionally, it upregulates gene expression, activating enzymes for NO synthesis (eNOS) and modulating molecular pathways that culminate in NO production [16]. GBE also decreases endothelin-1, a molecule with vasoconstrictive properties, and, systemically, GBE’s regulation of vasoactive substances increases both systolic and diastolic peak velocity measures [17]. Abnormal hemorheological properties contribute to the development of microvascular diseases such as microangiopathies seen in diabetes mellitus (DM). GBE impacts the hemorheological properties of blood by promoting erythrocyte deformability, and improving blood viscosity. GBE has a strong fibrinolytic effect equivalent to that of streptokinase, decreasing fibrinogen levels important for clotting. These changes improve blood perfusion, as shown by increased blood flow rates in the retinal capillaries of patients with diabetic retinopathy by altering hemorheological blood parameters [18]. Our ophthalmic rheography study demonstrated that a one-month course of Vasavital monotherapy resulted in (1) an increase in ocular pulse blood filling by 27.7% and 17.3%, respectively in patients with non-proliferative and preproliferative diabetic retinopathy, and (2) a decrease in vascular tone in large-caliber vessels by 8% in the former patients. Cytokines are major mediators of signal transduction during the inflammatory cascade. GBE decreased MIP-2 and MCP-1 cytokine levels, resulting in an anti-inflammatory effect. Zhang and colleagues [19] showed that GBE suppresses cytokine signaling 2, which further suppressed the inflammatory response. GBE suppresses PGE2 levels by downregulating cyclooxygenase-2 (COX-2) expression, which is responsible for producing prostaglandins from arachidonic acid [20]. Flavonoids have free radical scavenging activity targeting reactive oxygen species (ROS). Terpenoids, however, inhibit free radical release. Decreasing circulating free radicals reduce lipid peroxidation, erythrocyte malonaldehyde levels, decrease endothelial adhesive properties, reducing membrane peroxidation, while preserving its fluidity and integrity [21]. Ginkgo biloba L. is a source of quercetin, which increases generation of antioxidants, thus inhibiting oxidative stress. After balancing oxidation and anti-oxidation, NO and eNOS bioavailability improved, reducing inflammation, autophagy, and apoptosis, and inhibiting the recruitment of monocytes by adhesion factors, thereby protecting endothelial cell function. By elevating high-density lipoprotein (HDL) cholesterol absorption capacity, quercetin increased HDL anti-oxidation and reduced lipid accumulation [22]. GBE is a multifunctional neuroprotective agent and decreases post-ischemia-reperfusion injury stress [23]. In addition, GBE led to blocking the mitochondrial apoptotic pathway [24]. The bilobalide and ginkgolide components of GBE antagonize gamma-amino butyric acid and glycine inhibitory receptors, inducing an overall excitatory effect. Global excitation ultimately strengthens synapse formation [25]. Flavonoids such as nicotiflorin, rutin, and quercetin increased retinal ganglion cell survival rate [26]. In the diabetic retina, cellular changes in retinal neurons occur before vision loss or diabetic retinopathy can be identified clinically. Experimental animal studies demonstrated that the amplitudes of visual evoked potential recordings were significantly increased in alloxan diabetic rats with GBE compared with the diabetics [27], and in alloxan diabetic rats treated with GBE the electroretinogram of the isolated retina had significantly greater amplitude than that observed in untreated rats [28]. The current study demonstrated that, after treatment with a course of Vasavital, the inner retinal and visual pathway function as assessed by EEPT improved by 18% and 7.7%, respectively, and axial bundle conductivity as assessed by CFPD improved by 28.2% and 24.7%, respectively, in patients with NPDE and PPDR. GBE, a commonly used dietary supplement, has been shown to act as an antioxidant and freeradical scavenger, a membrane stabilizer, an inhibitor of the platelet-activating factor, a vasodilator, and a regulator of metabolism. Today, there exist a growing number of clinical studies on the application of GBE in cardiovascular disease, peripheral vascular disease and diabetic vascular complications [29]. The current study demonstrated that Vasavital contributed to the improved function of the central retina and fotopic afferent system in general, and the function of the photopic afferent system as assessed by light sensitivity at minutes 0 to 7 improved by 33.3%-40% in patients with NPDR and by 27.2%-33.3% patients with PPDR. Therefore, pharmaceuticals containing a combination of vitamins and polyphenol compounds exert a metabolic and antioxidant effect and improve the hemorheological properties of blood, thus improving the capacity for supplying blood to the visual tissue. One of such pharmaceuticals is Vasavital®, which contains not only GBE, but also a set of vitamins (thiamine, riboflavin, pyridoxine, ascorbic acid, rutin, and nicotinic acid) and bee pollen pellet. A one-month Vasavital course administered to patients with DR had a positive effect on the function of the visual system and ocular circulation parameters, which provides grounds for the use of the Ginkgo biloba-based preparation as a monotherapy or as part of a combined treatment for initial functional changes in the visual system in DR.
References 1.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016 387;1513–30. 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019 Nov;157:107843. 3.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007 Jul-Aug;14(4):179-83. 4.World Health Organization. Blindness and vision impairment. World Health Organization. February 26, 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-im... 5.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012 Mar; 35(3):556-64. 6.Li C, Miao X, Li F, Wang S, Liu Q, Wang Y, et al. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxid Med Cell Longev. 2017;2017:9702820.Croossref PubMed 7.Bucolo C, Marrazzo G, Platania CB, Drago F, Leggio GM, Salomone S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J Diabetes Res. 2013;2013:432695. 8.Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, et al. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid Med Cell Longev. 2019 Feb 12; 2019:9783429. 9.Srinivasan K. Chapter 42 - polyphenols in vision and eye health. In: Preedy V. R., editor. Handbook of Nutrition, Diet and the Eye. San Diego, CA, USA: Academic Press; 2014. pp. 413–421. 10.Bucolo C, Marrazzo G, Platania CB, Drago F, Leggio GM, Salomone S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J Diabetes Res. 2013;2013:432695. 11.Ponomarchuk VS, Konovalova NV, Khramenko Ni. Clinical experience of the use of Vasavital® in dry age-related macular degeneration and ischemic optic neuropathy. J Ophthalmol (Ukraine). 2021;1:38-45. 12.Pasyechnikova NV, Naumenko VO, Zborovska OV. [Clinical classification of and laser strategy for diabetic macular edema]. Odeskyi medychnyi zhurnal. 2009; 116 (6):77-9. Ukrainian. 13.Ponomarchuk VS. [Diagnostics using electrical phosphenes in ophthalmology]. Odesa: Astroprint; 2018. Russian. 14.Ritch R. Potential role for Ginkgo biloba extract in the treatment of glaucoma. Med Hypotheses. 2000 Feb;54(2):221-35. 15.Labkovich M, Jacobs EB, Bhargava S, Pasquale LR, Ritch R. Ginkgo Biloba Extract in Ophthalmic and Systemic Disease, With a Focus on Normal-Tension Glaucoma. Asia Pac J Ophthalmol (Phila). 2020 May-Jun;9(3):215-225. 16.Koltermann A, Hartkorn A, Koch E, Fürst R, Vollmar AM, Zahler S. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007 Jul;64(13):1715-22. 17.Wu YZ, Li SQ, Zu XG, Du J, Wang FF. Ginkgo biloba extract improves coronary artery circulation in patients with coronary artery disease: contribution of plasma nitric oxide and endothelin-1. Phytother Res. 2008 Jun;22(6):734-9. 18.Huang SY, Jeng C, Kao SC, Yu JJ, Liu DZ. Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr. 2004 Aug;23(4):615-21. 19.Zhang Y, Liu J, Yang B, Zheng Y, Yao M, Sun M, et al. Ginkgo biloba Extract Inhibits Astrocytic Lipocalin-2 Expression and Alleviates Neuroinflammatory Injury via the JAK2/STAT3 Pathway After Ischemic Brain Stroke. Front Pharmacol. 2018 May 16;9:518. 20.Ilieva I, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, et al. The effects of Ginkgo biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2004 Aug;79(2):181-7. 21.Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008 Jan;73(1):R14-9. 22.Song L, Zhang J, Lai R, Li Q, Ju J, Xu H. Chinese Herbal Medicines and Active Metabolites: Potential Antioxidant Treatments for Atherosclerosis. Front Pharmacol. 2021 May 13;12:675999. 23.Wu C, Zhao X, Zhang X, Liu S, Zhao H, Chen Y. Effect of Ginkgo biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet Mol Res. 2015 Jun 11;14(2):6387-94. 24.Zhao M, Wang XX, Wan WH. Effects of the ginkgo biloba extract on the superoxide dismutase activity and apoptosis of endothelial progenitor cells from diabetic peripheral blood. Genet Mol Res. 2014 Jan 14;13(1):220-7. 25.Watanabe CM, Wolffram S, Ader P, Rimbach G, Packer L, Maguire JJ, et al. The in vivo neuromodulatory effects of the herbal medicine ginkgo biloba. Proc Natl Acad Sci USA. 2001 Jun 5;98(12):6577-80. 26.Nakayama M, Aihara M, Chen YN, Araie M, Tomita-Yokotani K, Iwashina T. Neuroprotective effects of flavonoids on hypoxia-, glutamate-, and oxidative stress-induced retinal ganglion cell death. Mol Vis. 2011;17:1784-93. 27.Apaydin C, Oğuz Y, Ağar A, Yargiçoğlu P, Demir N, Aksu G. Visual evoked potentials and optic nerve histopathology in normal and diabetic rats and effect of ginkgo biloba extract. Acta Ophthalmol (Copenh). 1993 Oct;71(5):623-8. 28.Doly M, Droy-Lefaix MT, Bonhomme B, Braquet P. Effet de l'extrait de Ginkgo biloba sur l'électrophysiologie de la rétine isolée de rat diabétique [Effect of Ginkgo biloba extract on the electrophysiology of the isolated retina from a diabetic rat]. Presse Med. 1986 Sep 25;15(31):1480-3. 29.Tian J, Liu Y, Chen K. Ginkgo biloba Extract in Vascular Protection: Molecular Mechanisms and Clinical Applications. Curr Vasc Pharmacol. 2017;15(6):532-548.
Disclosures Corresponding author: Nataliia Khramenko, khramenkon@gmail.com Disclaimer: The views expressed in this article are those of the authors Source of support: Vazavital® was provided by the Ukrainian Pharmaceutical Company. Conflict of Interest Statement: All authors have no conflict of interest
|