J.ophthalmol.(Ukraine).2022;3:10-17.
|
http://doi.org/10.31288/oftalmolzh202231017 Received: 11.04.2022; Accepted: 27.04.2022; Published on-line: 15.06.2022 A prospective comparative study of the effect of ocular digital compression and non-compression following peribulbar anaesthesia on visual acuity and pupillary reactions Ajay Sharma, Head of Department; M. Laalasa, T. Krishna Sankar Foundation Eye Institute; Department of Vitreoretinal Services; Naiduthota, Visakhapatnam, Andhra Pradesh (India) TO CITE THIS ARTICLE:Ajay Sharma, Laalasa M., Krishna T. A prospective comparative study of the effect of ocular digital compression and non-compression following peribulbar anaesthesia on visual acuity and pupillary reactions. J.ophthalmol.(Ukraine).2022;3:10-7. http://doi.org/10.31288/oftalmolzh202231017 Background: Peribulbar lignocaine anaesthesia is commonly used in ophthalmic surgeries. Aim. To identify the effect of ocular digital compression (ODC) following peribulbar anaesthesia (PBA) on the optic nerve. Setting. A hospital-based prospective non-controlled observational study done at Sankar Foundation Eye Institute, Visakhapatnam, India. Method. This study conducted on 124 patients aged ≥40 years with non-complicated cataract. All patients were randomly divided into two groups by computer generated database; group 1 for PBA with ocular digital compression and group 2 for PBA without compression. The patient's eye was dilated, administered 2% lignocaine hydrochloride 5 mL, 0.75% bupivacaine 5 mL, and hyaluronidase 75 IU/mL injection for the peribulbar block(PBA). Vision and relative afferent pupil defect (RAPD) reactions were noted at 1 min and 10 mins after anaesthesia in both groups. Results. Out of 61 (PBA+ODC) cases, vision drop was seen in 33 (54.09%) after 1 min, and 28 (45.91%) patients has no change in the vision after 1 min, and 10 min, and 29 (47.54%) cases had RAPD after 1min, 32 (52.45%) cases had RAPD after 10 min, 32 (52.45%) cases had NSRL after 1min, 29 (47.5%) cases had NSRL after 10 min. This implies during 10 min compression, there is an appearance of RAPD in the previously NSRL eye. Out of 63 patients of PBA without ODC group, vision drop is seen in 48 (76.19%) after 1 min and 10 min. In this group, 57(90.4%) cases had RAPD after 1 min. and 56 (88.8%) cases had RAPD after 10min. 6 (9.5%) cases had NSRL after1 min. 7 (11.1%) cases had NSRL after 10 min. Males had more vision drop in the compression group. 33 (54.09%) cases in group 1 and 48 (76.19%) cases in group 2 had vision drop at 1min and 10 min. In compression group, 29 (47.5%) cases had RAPD 1 min, and 32 (52.45%) cases had RAPD at 10 min. In the non-compression group, 57 (90.47%) cases had RAPD at 1min, and 56 (88.88%) had RAPD at 10 min. The age of >60 yrs patients had more vision drop and RAPD irrespective of compression than the 40-60 yrs of age group, which shows that vascular changes may contribute to decreased optic nerve perfusion. Conclusion. The non-compression group had more vision drop and RAPD than the compression group. Age of >60 yrs and males had more percentage of vision drop. Кey words: peribulbar anaesthesia, relative afferent pupil defect, vision drop
Introduction The ideal anaesthesia aims to provide satisfactory pain relief during surgery and postoperatively and be easy to administer, and have no or minimal adverse effects. Cataract patients are operated on majorly under local anaesthesia through infiltration, topical, and intracameral. The infiltration is given as retrobulbar or peribulbar or sub-Tenon's block. Carl Koller first investigated cocaine as a topical anesthetic for eye surgery in 1884 [1]. Herman Knapp first used cocaine for retrobulbar anesthesia in 1884 [2]. Complications of retrobulbar anesthesia are retro and peribulbar hemorrhage, ocular penetration and perforation, retinal vascular spasm, optic nerve injury, and ocular myotoxicity [3]. In an audit of 12,000 retrobulbar blocks, Edge and Nicoll found that patients with the acquired vascular disease were at greater risk of periocular bleeding. In contrast, people with diabetes presented only a marginal risk of severe haemorrhage [4]. Peribulbar anaesthesia was discovered late in the 1980s by David and Manda [5]. The anaesthetic solution is injected into the extraconal compartment of the eye. Thus, most of the complications associated with retrobulbar anaesthesia are avoided [6]. Peribulbar anesthesia with lignocaine or bupivacaine is safer than retrobulbar anaesthesia for the optic nerve. The extraconal position of the PBA injection and the use of a shorter needle means there is less chance of complications such as optic nerve damage or scleral perforation [7, 8] Current study identifies peribulbar anaesthesia on optic nerve conduction and the effect of ocular digital compression following peribulbar block on optic nerve conduction. The primary outcome measures were vision drop and relative afferent pupil defect. Patients and methods Study design. This is a hospital-based prospective non-controlled observational study. Sample size: Patients who visited the general ophthalmology in Sankar Foundation Eye Institute, Visakhapatnam, India from June 2019 to July 2020 and who satisfied the inclusion criteria were selected for the study. The sample size was confirmed by the formula:
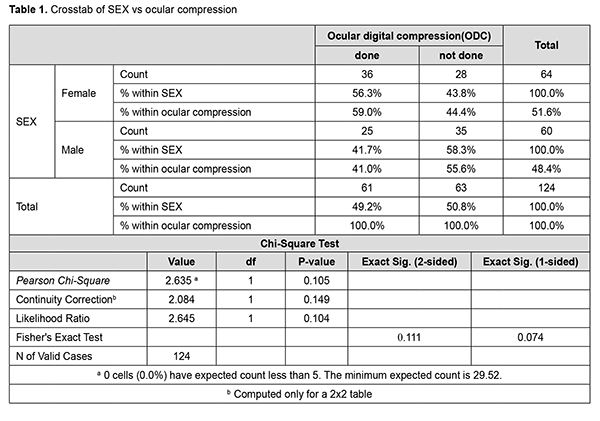
N = z12 1 - α/2 p (1-p) d Where, p = expected proportion; d = absolute precision; 1 - α/2 = desired confidence level; P = 0.6; Preci d=10; 1-α/2 = 95. Required sample (N) = 124 Inclusion criteria are patients undergoing cataract surgeries; patients who had no history of previous ocular surgeries; patients with normal pupillary reactions. Exclusion criteria are patients with diabetic retinopathy, patients with any neuro-ophthalmology disorder, any disease involving the retina & optic nerve, patients with a history of Glaucoma, coexisting ocular pathologies, and complicated cataracts are excluded. Method. All cases that met inclusion criteria were taken, and informed written consent was obtained. The same investigator recorded all the variables in the study to minimize bias. We divided the patients into two groups: peribulbar block given with no ocular digital compression, and the second group was given ocular compression followed after PBA. Examination. The patients' visual acuity was measured with a standard Snellen chart at 10 feet, and an afferent pupil defect is examined using the reverse swinging Marcus Gunn test, with a bright pen torch, binocular loupe. The eye was dilated with phenylephrine 5%w/v, and tropicamide 0.8% w/v. Dosage. We used 10 ml injection, out of which 5 ml is lidocaine, 5ml is bupivacaine, and 75 units of hyaluronidase. The patient received 2% lignocaine hydrochloride 5 mL (LOX 2%; Neon Pvt. Ltd, Mumbai, India), 0.75% bupivacaine 5 mL (ANAWIN 0.5%; Neon Pvt. Ltd, Mumbai, India), and hyaluronidase 75 IU/mL injection (Hynidase; Shreya Life Sciences, Aurangabad, India) for the peribulbar block. The needle was first inserted with 5 ml of local anaesthetic solution through the eyelid at a point between the lateral third and medial two-thirds of the lower orbital margin, with the bevel facing the globe. The second injection was given in the superomedial compartment. The needle was introduced through the upper lid at about 2 mm medial and inferior to the supraorbital notch. The needle was then advanced in a sagittal plane under the orbit roof for a maximal depth of 25 mm, where the remaining 5 mL of local anaesthetic was injected at a similar rate as for the inferior injection. Injection of the local anaesthetic was stopped when there was either firmness in the globe or any resistance to further depressing the syringe's plunger. For group 1, the globe was compressed gently with the middle three fingers placed over the sterile gauze pad on the upper eyelid with the middle finger pressing directly down on the eyeball. Every 30 seconds, digital pressure was released for 5 seconds to allow for the vascular pulsations to occur. Following this compression, vision and pupillary reactions were noted after 1 min, and 10 mins post anaesthesia. For group 2, the globe was not compressed. Visual acuity and RAPD were measured in both groups. Routine monitoring for all patients included electrocardiogram, non-invasive arterial pressure monitoring, and pulse oximetry. Both the block and digital compression were performed by an ophthalmologist (primary investigator) throughout the study. Statistical analysis. The results were recorded using spreadsheets, statistical analysis performed by software SPSS (Statistical Package for the Social Science) software version 22. Descriptive measures like mean, standard deviation, and range were calculated for all parameters. Analysis was performed using paired t-test, unpaired t-test, and chi-square test. A p-value ≤of 0.05 has been considered statistically significant. Ethical consideration. The authors confirm that ethical approval was obtained for the study from Sankar Foundation Eye Institute Ethics Committee, Visakhapatnam, India. Results Eighty-seven cases belonged to 40-60 years, and 37 cases belonged to > 61 years of age groups. There were 64 (51.61%) female and 60 (48.3%) male patients included (Table 1). Among the 40-60 years of age group, 40 (45.97%) patients were given PBA and ODC, and another 47 (54.02%) patients were given PBA only without ODC. Out of 37 patients, 21 (56.75%) cases were given PBA and ODC, and 16 (43.24%) were given PBA only without ODC. Out of 64 female patients, 36 (56.3%) subjects were given PBA and ODC and 28 (43.8%) cases were given PBA only without ODC. Out of 60 male patients, 25 (41.7%) were given PBA and ODC, and 35 (58.3%) were given PBA only without ODC (Table 2).
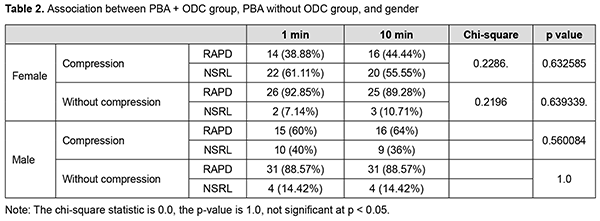
Effect of PBA + Ocular digital compression (ODC) on pupillary reactions Out of 64 females, 36 were given ocular compression, 28 were without compression (Table 3). After 1 min post anaesthesia, 14 (38.88%) cases had RAPD and 22 (61.11%) cases among of 36 had NSRL. After 10 minutes post anaesthesia, 16 (44.44%) had RAPD and 20 (55.55%) among of 36 had NSRL.
Effect of PBA + without compression on pupillary reactions Out of 64 females, 28 patients were given only PBA without ODC. After 1 minute post anaesthesia, 26 (92.85%) cases had RAPD, and 2 (7.14) cases had NSRL. After 10 minutes post anaesthesia, 25 (89.28) subjects had RAPD, and 3 (10.71%) cases had NSRL. Out of 60 male subjects, 25 patients were given PBA and ODC, 35 cases were given PBA only without ODC. After 1 minute post anaesthesia, 15 (60%) had RAPD, and 10 (40%) had NSRL among of 25 in group 1. After 10 minutes post anaesthesia, 16 (64%) had RAPD, and 9 (36%) had NSRL in group 1. Out of 60 males and 35 cases were given PBA only without ODC. In these 35 cases of group 2, after 1 minute post anaesthesia, 31 (88.57%) had RAPD, and 4 (14.42%) had NSRL. After 10 minutes post anaesthesia, 31 (88.57%) had RAPD and 4 (14.42%) had NSRL in group 2 (Table 4).
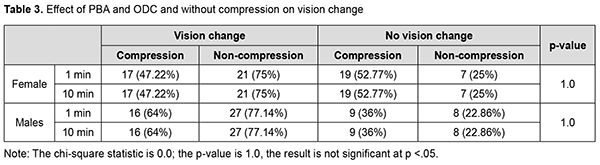
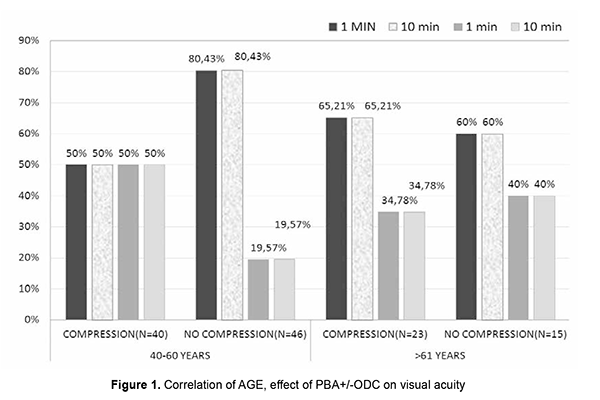
Association of PBA + ODC and PBA without ODC on vision change Out of 64 females, 36 cases were given PBA+ODC and 28 cases were given PBA only + without ocular compression. 17 (47.22%) subjects had vision drop among 36 female patients in the compressive group. Out of 28 non-compressive females, 21 (75%) had a drop in the vision and 7 (25%) had no change in the vision after 1 min and 10 min post anaesthesia. Out of 60 males, 25 cases were given PBA+ODC and 35 cases were given PBA only without compression. 16 (64%) subjects had vision drop after 1minute and 10 min postanaesthesia among compressive male patients. 27 (77.14%) cases had vision drop after 1minute and 10-minute post anaesthesia among non-compressive male patients (Fig. 1).
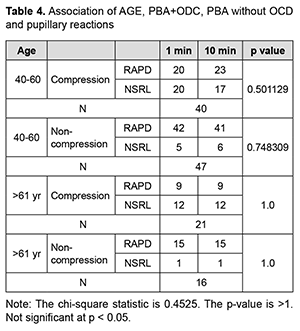
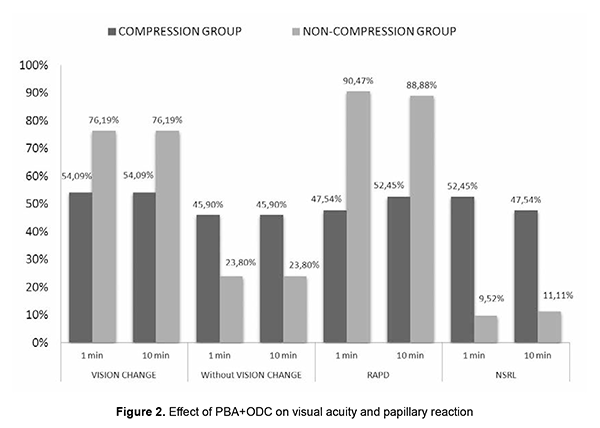
Out of 40 patients of the PBA+ODC group of 40-60 yrs age group, 20 cases had RAPD, 20 cases had NSRL in 1 min, 23 cases had RAPD, 17 cases had NSRL after 10 min. This indicates that after 10 minutes of compression, three patients developed RAPD from the NSRL batch. Out of 37 patients of >61 yrs., 21 cases were given PBA+ODC and 16 cases were given PBA only without ODC. In the compression group, out of 21 patients, 9 cases had RAPD, and 12 cases had NSRL after 1 min and 10 min. Out of 37 patients of>61 yrs age, 16 cases were given PBA only without ODC, 15 cases had RAPD, and 1 case had NSRL after 1 min and 10 min. In our study, out of 124 patients, 61 cases were given by PBA+ODC, PBA gave 63 cases only without compression. Out of 61 (PBA+ODC) cases, vision drop was seen in 33 (54.09%) after 1min, and 28 (45.91%) patients have no change in the vision after 1 min and 10 min. Out of 61 patients (PBA+ODC) cases, 29 (47.54%) subjects had RAPD after 1min, 32 (52.45%) subjects had RAPD after 10 min, 32 (52.45%) cases had NSRL after 1 min, 29 (47.5%) cases had NSRL after 10 min. In the PBA+ODC group, 54.09% had vision drop. These 29 cases had RAPD after 1 min, 32 patients had RAPD after 10 min. Thirty-two cases had NSRL after 1min, 29 cases had NSRL after 10 min. This implies during 10 min compression, there is an appearance of RAPD in the previously NSRL eye (Fig. 2).
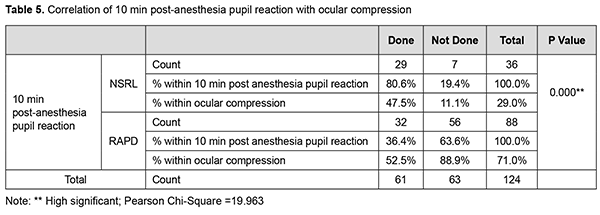
Out of 63 patients of PBA without ODC group, vision drop is seen in 48 (76.19%) after 1 min and 10 min. 15 (23.8%) cases had no change in the vision after 1min and10 min. Out of 63 patients of PBA without ODC group, 48 (76.19%) subjects had vision drop after 1 min and 10 min. 15 (23.8%) subjects had no change in the vision after 1 min and 10 min. Out of 63 patients of PBA without ODC group, 57 (90.4%) cases had RAPD after 1 min. and 56 (88.8%) cases had RAPD after 10 min. 6 (9.5%) subjects had NSRL after 1 min. 7 (11.1%) case had NSRL after 10 min. Out of 63 patients of PBA without ODC group, 76.19% had significant vision drop. 57(90.47%) cases had RAPD after 1 min, 56 cases had RAPD at 10 min. This implies 1 of the RAPD case showed NSRL after 10 mins of PBA without compression (Table 5). Discussion Ropo et al.[9] has demonstrated by computed tomography that solution injected into the retrobulbar space becomes extraconal 3-8 min following injection, and that peribulbar injection reaches intraconal space 2-6 min later; this suggests that a smaller increase in orbital volume by RBA can nevertheless cause a comparable increase in IOP to that associated with the larger peribulbar injection by temporary entrapment of solution within the intraconal space. This would also imply that the IOP increase at 5 min would be less following retrobulbar injection, with dissipation of smaller volume into extraconal tissues. Our study aimed to study the effect of PBA+ODC and PBA without ODC on visual acuity and pupils after 1 and 10 mins. We used large volumes of injection (10ml). There might be early diffusion of injection volume into the intraconal space, hence the rise in IOP, [10-12] decreased POBF, decreased retinal PP, and decreased optic nerve conduction. IOP rise could be attributed to the agent volume used and orbital volume and tightness in the orbital septum [13]. Association of Age, PBA+ODC and visual acuity In the compression group of 40-60 yrs age, 50% of patients had vision drop. In the non-compression group in the 40-60 yrs age group, 80% of patients had vision drop. In the compression group of >61 yrs age, 65% had vision drop. In the non-compression group of >61 yrs, 60% had vision drop. There was a change in the visual acuity with differences in age group, but as the other variable like ODC was present, we cannot solely judge the change in the visual acuity was due to age differences. As we were using the same volume of anaesthetic solution in all the individuals, compression application significantly affects the vision. This vision drop in the Compressive group (40-60 yrs) may be due to ODC is not standardized, pressure exerted could be presumably high and caused reduced ciliary perfusion pressure [14]. Vision drop in the non-compressive group (40-60 yrs) can be explained by volume-related compression effect on the optic nerve directly or by reducing POBF. From the above data, it was noted that vision drop in age group >60 yrs is almost similar in both compression and non-compression groups. This can be explained by vascular changes (atherosclerosis) in the vessels and so reduction in POBF in ciliary arteries. Mostafa et al. studied the effect of 2 volumes of RBA (2.5 ml and 4 ml). the significant difference regarding globe akinesia with a large volume of anaesthesia and concluded that efficacy was not affected by the patient's age [15]. These findings are not collating with our study results. In our study, in >61 yrs group, vision drop was seen in both the compression (65%) and non-compression (60%) groups. This vision drop in the compression group is more in >61 yrs age group when compared to 40-60 yrs age group. Association of Age, PBA+ODC, and pupil reaction In our study, in 40-60 yrs age in PBA+ODC group, 20 cases had RAPD at 1 min, 23 cases had RAPD at 10 min, 20 cases had NSRL at 1 min, and 17 cases had NSRL at 10 min. respectively. This implied that during the block's course, three patients' normal pupil reaction got converted to RAPD. This might be because of various factors like ocular compression, orbital volume, the anaesthetic agent itself, and the volume of anaesthesia. Ocular compression may result in an additional, albeit temporary, hypertensive effect, which causes a rise in IOP that causes decreased ocular perfusion pressure. The IOP rise is also attributed to the volume of agent, orbital volume, and tightness of orbital septa. Gender and PBA without ODC Out of 64 females, 36 were given PBA+ODC and 28 were given PBA without ODC. Out of 36 in the PBA+ODC group, 17 cases had vision change. Out of 28 in PBA without ODC group, 21 cases had vision change. Our study observed 47.22% had vision change in PBA+ODC,75% in non-ODC group, implied in compression group vision is dropped might be due to large volume used in the study despite compression. In the non-compressive group, 75% had vision drop and indicated a volume-related compressive effect on the optic nerve. The same findings have been noticed at 1min, 10 min postanaesthesia. that implied effect will persist for 10mins. Ropo et al. [16] recorded a rise of 25% IOP following PBA of 7-12 ml at 10 min. Ropo et al. demonstrated by CT that solution injected into retrobulbar space becomes extraconal 3-8 min following injection, that peribulbar injection reaches the intraconal space 2-6 min later, but as the volume injected here is 10 ml it might enter intraconal space earlier and so caused vision drops and pupillary reaction changes at 1min, and 10 min. Out of 64 female patients, in 36 PBA+ODC cases, 14 had RAPD at 1 min and 16 had RAPD at 10 min. Twenty-two cases had NSRL at 1 min and 20 cases had NSRL at 10 min. In 28 cases of PBA without ODC group, 26 patients had RAPD at 1 min and 25 cases had RAPD at 10 min. 2 cases had NSRL at 1 min and 3 cases had NSRL at 10 min. We observed that in the non-compression group, RAPD noticed more than that seen in the compressive group because of the volume-related compressive effect. With compression, two patients' NSRL got converted to RAPD due to ocular compression's temporary hypertensive effect. Male and PBA with/without ODC Out of 60 cases, 25 were given PBA+ODC, and 35 were not given compression. Out of 25 in the compression group, 16 (64%) cases had vision change. Out of 35 cases in the non-compression group, 27(77.14%) cases had vision change. Out of 25 cases in the PBA+ODC group, 15 had RAPD at 1 min and 16 had RAPD at 10 min. 10 cases had NSRL at 1 min, and 9 had NSRL at 10 min. Out of 35 cases in the non-compression group, 31 cases had RAPD at 1 min, 31 cases had RAPD at 10 min, 4 cases had NSRL at 1 min, and 4 cases had NSRL at 10 min. Vision drop and RAPD are more in the non-compressive group. Various factors attribute to the effect, such as speed of injection, interindividual variation in orbital volume, compliance, the resistance of orbital septum to orbital volume increase, and vascular effects. The study demonstrates that cases without compression had maximum vision changes and pupil reaction changes irrespective of gender. Out of 61 cases of the PBA + ODC compression group, 33 cases had vision drop, and 28 cases had no vision change. Twenty-nine cases had RAPD at 1 min, and 32 cases had RAPD at 10 min in the compression group. Peribulbar anaesthesia causes rise in IOP depending on the volume of anaesthesia given/depending on orbital volume and compliance or speed of injection or tightness of orbital septum and also depends upon the agent used in the anaesthetic mixture. Our study used 10 ml injection, out of which 5 ml is lidocaine, 5 ml is bupivacaine, 75 units of hyaluronidase, and adrenaline. Jay et al. used 3, 4 and 5 ml and showed no difference in IOP rise. Donoghue et al. [17] showed 6.2 mm Hg rise of IOP from baseline at 1 min and 5 min without compression, and they noticed a 3.6 mmHg rise from baseline. An increase in IOP is presumably secondary to orbital pressure caused by large volumes of anaesthetic injected. Bupivacaine impairs nitric oxide-mediated vasodilatation [18], lidocaine reduces ocular pulse volume, wich maybe due to paradoxical vasoconstriction caused by high concentration of anaesthetic agent [19] (adrenaline also causes lowered ciliary perfusion, reduced ciliary blood flow) [20] Hyaluronidase added to the injections of lidocaine appears to lessen the reduction of POBF by reducing the volume effect of anaesthetic mixture [21]. Peribulbar anaesthesia may cause a rise in IOP depending on the volume of anaesthesia given/depending on orbital volume and compliance or speed of injection or tightness of orbital septum upon the agent used in the anaesthetic mixture. Our study used 10 ml of injection, out of which 5 ml is lidocaine, 5 ml is bupivacaine, 75 units of hyaluronidase, and adrenaline. Jay et al. used 3, 4, and 5 ml and showed no difference in IOP rise. Palay et al. [22] showed 6.2 mm Hg after 4 ml of RBA; Ropo et al. reported a rise of 25% following 7-12 ml of injection. Donoghue et al. showed a 6.2 mm Hg elevation of IOP from baseline at 1 min, and at 5 min without compression, he noticed a 3.6 mm Hg rise from baseline. An increase in IOP is presumably secondary to a rise in orbital pressure caused by large volumes of anaesthetic injected. There might be reduction of POBF following PBA with and without ODC, with the 10 ml of anaesthetic mixture, there will be raise in the intraorbital pressure transmitted as intraocular pressure, which consequently reduces perfusion pressure of optic nerve and so ocular blood flow [23] will be reduced and so optic nerve conduction. Because the anaesthetic effect of PBA on the optic nerve can also directly affects visual acuity and pupils. Peribulbar anaesthesia, using a relatively large local anaesthetic solution, produces a significant effect on optic nerve function as detected by visual acuity and afferent pupil defect. The limitations of the study are that Ocular digital compression has not been standardized. Old age people could have been excluded as vascular caliber changes play a significant role. Orbital volume and size could not be selected and standardized (interindividual variability). IOP monitoring could have also included in the study. Conclusion Out of 61 patients of the PBA+ODC group, a vision drop was seen in 33 (54.09%) after 1min, and 28 (45.91%) patients have no change in the vision after 1 min and 10 min. Out of 63 patients in the non-compression group, 48 (76.19%) had vision drop. Irrespective of age and gender, the non-compression group had more vision drop and RAPD when compared to the compression group. There is no significant change in the 40-60 yrs age group, but >60 yrs age group had more vision drop. Males had more vision drop in the compression group. Four cases of NSRL turned to RAPD at 10 min postanaesthesia. Examining for a relative afferent pupil defect appears to be a valuable method for predicting which patients may be likely to see through the operated eye preoperatively. What is new? This is a Well-designed observational study with adequate sample size is the first prerequisite for the successful conduct of a study. We were able to attain a reasonable number of cases to assess the effect of peribulbar anaesthesia & ocular digital compression on optic nerve conduction: fixed volume, fixed anaesthetic agents, and technique employed by the same candidate for all individuals.
References 1.Redman M. Cocaine: What is the crack? A brief history of the use of cocaine as an anesthetic. Anesth Pain Med 2011;1:95-7. 2.Jaichandran V. Ophthalmic regional anaesthesia: A review and update. Indian J Anaesth 2013;57:7-13. 3.Palte HD. Ophthalmic regional blocks: management, challenges, and solutions. Local Reg Anesth 2015; 8: 57-70. 4.Edge KR, Nicoll JM. Retrobulbar hemorrhage after 12,500 retrobulbar blocks. Anesth Analg 1993; 76: 1019-1022. 5.Davis DB 2nd, Mandel MR. Posterior peribulbar anesthesia: an alternative to retrobulbar anesthesia. J Cataract Refract Surg 1986;12:182-4. 6.http://eyewiki.aao.org/Ocular_Anesthesia (accessed on 26th December 2016) 7.Pautler SE, Grizzard WS, Thompson LN, Wing GL. Blindness from retrobulbar injection into the optic nerve. Ophthalmol Surg 1986; 17: 334-7. 8.Ramsey RC, Knobloch WH. Ocular perforation following retrobulbar anaesthesia for retinal detachment surgery. Amj Ophthalmol 1978; 86: 61-4. 9.Ropo A, Ruusuvaara P, Setala K. Visual evoked potentials after retrobulbar or periocular anaesthesia. BrJ Ophthalmol1992; 76: 541-4. 10.Jay WM, Carter H, Williams B, Green K. Effect of applying the Honan intraocular pressure reducer before cataract surgery. AmJr Ophthalmol 1985; 100: 523-7. 11.Palay DA, Stulting RD. The effect of ocular compression on intraocular pressure following retrobulbar anaesthesia. Ophthalmic Surg 1990; 21: 503-7. 12.Ropo A, Ruusuvaara P, Paloheimo M, Mauuksela E-L, Nikki P. Effect of ocular compression. (Autopressor) in intraocular pressure in periocular anaesthesia. Acta Ophthalmol (Copenh) 1990; 68: 227-9. 13.Morgan J E, Chandna A. Intraocular pressure after peribulbar anaesthesia, is the Honan's Balloon necessary? Br J Ophthalmol 1995; 79(1): 46-4. 14.Ubah J, Ajayi BJ, Bekibele CO. Comparison of fixed weight and digital massage techniques for intraocular pressure reduction after peribulbar anaesthesia. Nigerian J Ophthalmol. 2004;12:60-4. 15.Mostafa EM, Mounir A. Effect of the volume of anesthetic solutions and patient's age on the efficacy of retrobulbar anesthesia. Delta Journal of Ophthalmology. 2018 Apr 1;19(2):87. 16.Ropo A, Ruu'suvaara P, et al. Effect of ocular compression on intraocular pressure in periocular anaesthesia. Acta Ophthalmol 1990;68:227-9. 17.O'Donoghue E, Batterbury M, Lavy T. Effect on intraocular pressure of local anaesthesia in eyes undergoing intraocular surgery. Br J Ophthalmol. 1994;78:6057. 18.Meyer P, Flammer J, Lüscher TF. Local anesthetic drugs reduce endothelium-dependent relaxations of porcine ciliary arteries. Investigative ophthalmology & visual science. 1993 Aug 1;34(9):2730-6. 19.Pianka P, Weintraub-Padova H, Lazar M, Geyer O. Effect of sub-Tenon's and peribulbar anesthesia on intraocular pressure and ocular pulse amplitude. Journal of Cataract & Refractive Surgery. 2001 Aug 1;27(8):1221-6. 20.Hessemer V, Heinrich A, Jacobi KW. Ocular circulatory changes caused by retrobulbar anesthesia with and without added adrenaline. Klinische Monatsblatter fur Augenheilkunde. 1990 Dec 1;197(6):470-9. 21.Hulbert MFG, Yang YC, Pennefather PM, et al. Pulsatile ocular blood flow and intraocular pressure during retrobulbar injection of lignocaine: influence of additives. J Glaucoma 1998;7:413–16. 22.Palay DA, Stulting RD. The effect of ocular compression on intraocular pressure following retrobulbar anaesthesia. Ophthalmic Surg 1990; 21: 503-7. 23.Watkins R, Beigi B, Yates M, Chang B, Linardos E. Intraocular pressure and pulsatile ocular blood flow after retrobulbar and peribulbar anaesthesia. Br.J.Ophthalmol. 2001;85(7):796-8.
Information about authors and disclosure of information Corresponding author: Ajay Sharma1, Sankar Foundation Eye Hospital Institute, Visakhapatnam, Andhra Pradesh, India. Authors’ contributions: All authors contributed equally to this work Funding information: This research does not received specific grant from any funding agency in the public, commercial or not-for -profit sector. Competing interests: The authors have declared that no competing interests exist. Abbreviations: ODC – ocular digital compression; PBA – peribulbar anaesthesia; RAPD – relative afferent pupil defect.
|