J.ophthalmol.(Ukraine).2022;2:42-47.
|
http://doi.org/10.31288/oftalmolzh202224247 Received: 13 February 2021; Published on-line: 30 April 2022 Morphological and functional changes in the rabbit iris and ciliary body in experimental hypopinealism O. V. Nedzvetska 1, U. A. Pastukh 1, E. V. Kihtenko 2, I. V. Pastukh 3, N. N. Sotnik 4, N. A. Goncharova 3, O. V. Kuzmina de Gutarra 1
1 Kharkiv Medical Academy of Post-Graduate Education; Kharkiv (Ukraine)
2 Kharkiv National Medical University; Kharkiv (Ukraine)
3 V. N. Karazin Kharkiv National University; Kharkiv (Ukraine)
4 SI "V. Danilevsky Institute for Endocrine Pathology Problems of the NAMS of Ukraine"; Kharkiv (Ukraine)
TO CITE THIS ARTICLE: Nedzvetska OV, Pastukh UA, Kihtenko EV, Pastukh IV, Sotnik NN, Goncharova NA, Kuzmina de Gutarra OV. Morphological and functional changes in the rabbit iris and ciliary body in experimental hypopinealism. J.ophthalmol.(Ukraine).2022;2:42-7. http://doi.org/10.31288/oftalmolzh202224247 Background: Previous morphological studies have found degenerative retinal abnormalities in experimental hypopinealism. It is important to determine the morphology and function of the iris and ciliary body in prolonged pineal gland dysfunction with melatonin deficiency.
Purpose: To determine the morphology and function of the iris and ciliary body in rabbits maintained under conditions of prolonged around-the-clock illumination leading to hypopinealism and melatonin deficiency.
Material and Methods: Fifty five adult rabbits (110 eyes) were used in this experimental study. Animals were divided into two groups, an experimental group of 32 animals maintained under conditions of around-the-clock illumination to induce functional hypopinealism, and a control group of 23 animals maintained under natural day/night cycle conditions. Both groups were subdivided into 5 subgroups based on the duration of the experiment: 1-2 months, 3-5 months, 8-12 months, 18-19 months, and) 26-28 months. Blood melatonin levels were assessed by an enzyme-linked immunosorbent assay. A comprehensive morphological study of rabbit iris and ciliary body specimens was conducted.
Results. Blood melatonin level at night time in the experimental group was almost six-fold lower than blood melatonin level in the control group. In animals maintained under conditions of around-the-clock illumination, marked circulatory abnormalities with markedly dilated and hyperemic vessels were observed in the iris and ciliary body at time points until 12 months. In addition, at 12 to 28 months, iris and ciliary body vascular circulatory abnormalities appeared to be changed by sclerotic abnormalities. In animals exposed to around-the-clock illumination, vascular sclerotic changes appeared substantially earlier, and were much more marked, than in control animals. The mean vascular wall thickness (VWT) in iris and ciliary body specimens for the experimental group was 1.5-fold higher than that for the control group (177.5 ± 7.3×10-6 m vs 101.9 ± 4.4×10-6; р < 0.05) at 18 to 19 months, and twice higher than that for the control group (217.4 ± 8.7×10-6 m vs 107.2 ± 5.2 ×10-6 m) at 26 to 28 months. The like newly formed rough bundles of collagen fibers found in an analogue of the Schlemm canal may exert a very negative effect on hydrodynamics of the eye.
Keywords: ciliary body, iris, around-the-clock illumination, hypopinealism, melatonin, morphological and functional changes
Introduction
The eye is directly involved in the regulation of circadian hormonal rhythms in humans and animals through retinohypotholamic and retinocortical connections. A natural day-night cycle (i.e., a daily alternation of the effects of intensive light and darkness on central nervous system structures) enables a rhythm of pineal gland melatonin production as well as the development of circadian rhythms [1-6]. At night blood melatonin levels are higher, whereas daylight suppresses melatonin production [2, 3, 5]. Pineal gland dysfunction with decreased melatonin production at night results in impaired physiological processes (e.g., cellular free radical processes) and contributes to pathological changes in different body tissues [7, 8]. It has been found that melatonin is produced in ocular tissues like the retina, ciliary processes and lens epithelium [9-13] and involved in aqueous humor production [14]. We have previously found early atherosclerotic and degenerative processes in the rabbit retina, similar to those seen in age-related macular degeneration (AMD), in the presence of experimental hypopinealism [15].
The purpose of this study was to determine the morphology and function of the iris and ciliary body in rabbits maintained under conditions of prolonged around-the-clock illumination leading to hypopinealism and melatonin deficiency.
Material and Methods
Fifty five adult rabbits were used in this experimental study which was conducted at the vivarium of the Danilevskii Institute for Endocrine Pathology Problems. All animal experiments were conducted in compliance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986), General Ethical Principles for Experiments in Animals, adopted by the First National Bioethics Congress (2001), and the Law of Ukraine on Protection of Animals from Cruel Treatment No. №3447-IV dated 21.02.2006.
To induce functional hypopinealism [7, 8], animals were maintained under conditions of around-the-clock illumination (natural sunlight at daytime and 30-40-lux illumination from 100-W incandescent lamps at night time). Illuminance in animal cages was measured with an IU-117 lux meter.
Animals were divided into two groups, an experimental group of 32 animals maintained under conditions of around-the-clock illumination and a control group of 23 animals maintained under natural day/night cycle conditions. Both groups were subdivided into 5 subgroups based on the duration of the experiment: (1) 1-2 months, (2) 3-5 months, (3) 8-12 months, (4) 18-19 months, and (5) 26-28 months. Blood melatonin levels were assessed by an enzyme-linked immunosorbent assay (ELISA) using commercially available kits (IBL International, Hamburg, Germany) to investigate hormonal activity of the pineal gland. Photometric measurements were performed on an ELISA plate reader (Stat Fax 303 Plus, Awareness Technology Inc, Palm City, FL).
All animals were euthanized by an overdose of sodium thiopental in accordance with the guidelines from the Ministry of Health of Ukraine [16].
The eyes had been enucleated, fixed in neutral formalin and routinely processed into paraffin-embedded blocks. Blocks were cut into 4-5-μm sections which were mounted on slides and stained with Mallory’s hematoxylin and eosin and van Gieson's picro-fuchsin (to show connective tissue). Thereafter, low-magnification microscopy was used for general assessment of the tissue of interest, and morphological study was conducted [17, 18]. Histological and morphometric evaluations were carried out under a light microscope Olympus BX-41 (Olympus Europe GmbH, Munich, Germany) using appropriate software (Olympus DP-Soft 3.1, Olympus Europe GmbH) and Microsoft Excel [19].
Morphometric analysis was conducted using the field method [20].
Analysis of variance and alternative analysis were employed for statistical analysis [21].
Results
At 1 month after initiation of the experiment, blood melatonin level at daytime in the experimental group decreased to 29.12 ± 5.85 pmol/l, which was statistically significantly lower than blood melatonin level in the control group (54.41 ± 6.15 pmol/l; р< 0.05). In addition, blood melatonin level at night time in the experimental group decreased to 62.26 ± 5.27 pmol/l, which was almost six-fold lower than blood melatonin level in the control group (369.45 ± 14.35 pmol/l; р< 0.05). This level of melatonin production was maintained at subsequent time points.
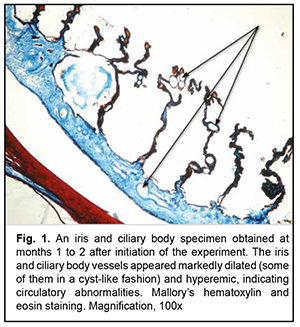
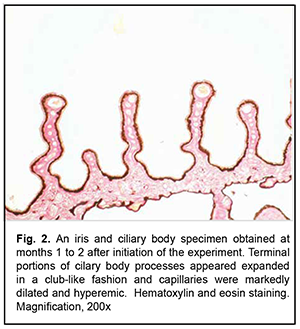
Low-magnification microscopy for general assessment of the iris and ciliary body specimens obtained at months 1 to 2 revealed markedly dilated and hyperemic vessels (small arteries and arterioles of the iris and small arterioles and capillaries of the ciliary body) in the experimental group specimens (Figs. 1 and 2). Of the cilary body capillaries, those of terminal processes appeared the most dilated and hyperemic (Fig. 2). Our morphometric study found that the mean relative vacular area (RVA) in iris and ciliary body specimens obtained at months 1 to 2 was 7.8 ± 0.27% for the experimental group and 4.5 ± 0.21% for the control group, with the difference between groups at this time point being statistically significant (р< 0.05, Table 1). In addition, the mean vascular wall thickness (VWT) in iris and ciliary body specimens obtained at months 1 to 2 was 59.2 ± 1.2×10-6 m for the experimental group, with no significant difference between groups at this time point (Table 1).
  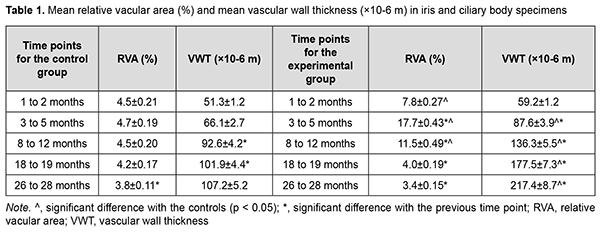 Low-magnification microscopy for general assessment of iris and ciliary body specimens obtained at months 3 to 5 revealed marked circulatory disorders in iris and ciliary body vessels for the experimental group (Fig. 3), and the morphometric study found these disorders to be increased compared to the previous time point. The mean RVA in iris and ciliary body specimens obtained at months 3 to 5 was 17.7 ± 0.43% for the experimental group, which was more than 3.5-fold higher than that for the control group (4.7 ± 0.19%, р< 0.05 ) (Table. 1).
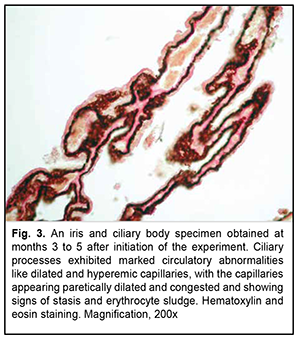 In is noteworthy that at 3 to 5 months, the most severe circulatory changes were found in terminal portions of cilary processes, with the capillaries appearing paretically dilated and congested and showing signs of stasis and erythrocyte sludge (Fig. 3). At this time point, the mean VWT in iris and ciliary body specimens for the experimental group was 87.6 ± 3.9×10-6 m, which was significantly larger than that for the control group (66.1 ± 2.7×10-6; р< 0.05) as well as that at the previous time point (59.2 ± 1.2×10-6 ; р< 0.05) (Table 1).
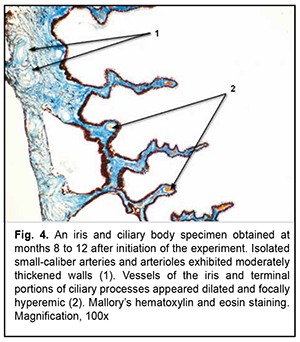
Low-magnification microscopy for general assessment of iris and ciliary body specimens obtained at months 8 to 12 revealed less severe circulatory disorders in iris and ciliary body vessels for the experimental group (Fig. 4) than those at the previous time points. At this time point, the mean RVA in iris and ciliary body specimens for the experimental group was 11.5 ± 0.49% m, which was significantly larger than that for the control group (4.5 ± 0.20%; р< 0.05) but less than that at the previous time point (17.7 ± 0.43%; р< 0.05) (Table 1). Aside of moderate signs of circulatory disorders, low-magnification microscopy for general assessment of iris specimens obtained at months 8 to 12 revealed small-caliber arteries with thickened walls (Fig. 4). Consequently, morphometric study found that the mean VWT in iris and ciliary body specimens for the experimental group was statistically significantly increased (136.3 ± 5.5×10-6 m, р< 0.05 ) (Table 1).
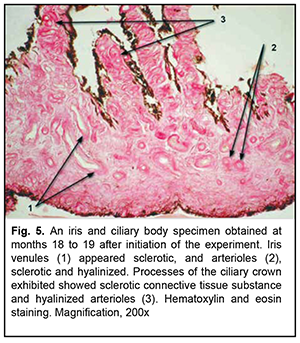
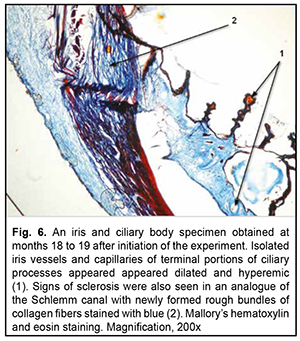
 At 18-19 months of the experiment, low-magnification microscopy for general assessment of iris and ciliary body specimens revealed markedly thickened and sclerotic vascular walls (Fig. 5) for the experimental group. Particularly, walls of not only small iris arteries, but also of small iris veins of showed sclerosis, and the ciliary body showed sclerotic connective tissue substance and hyalinized capillaries. At this time point, the mean VWT in iris and ciliary body specimens for the experimental group was 177.5 ± 7.3×10-6 m (Table 1), which was significantly larger than that for the control group (101.9 ± 4.4×10-6; р< 0.05) as well as that at the previous time point (136.3 ± 5.5×10-6 ; р< 0.05). Signs of sclerosis were also seen in an analogue of the Schlemm canal with newly formed rough bundles of collagen fibers (Fig. 6).
  Signs of vascular dilation and other circulatory disappeared at 18-19 months of the experiment, with the mean RVA in iris and ciliary body specimens for the experimental group (4.0 ± 0.19%) being significantly lower than that at the previous time point (11.5 ± 0.49%; р< 0.05) and practically the same as that for the control group (4.2 ± 0.17%; р> 0.05).
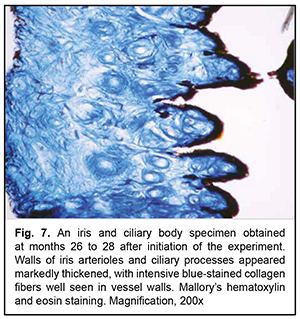
At 26-28 months of the experiment, there was sclerosis of the connective tissue proper, with markedly sclerotic small arteries and veins and glassy degeneration of arterioles (Fig. 7), for the iris and ciliary body specimens of the experimental group. In addition, the mean VWT in iris and ciliary body specimens for the experimental group was 217.4 ± 8.7×10-6 m (Table 1), which was significantly larger than that for the control group and that at the previous time point (р< 0.05). However, in spite of extensive vascular sclerosis, the mean RVA in iris and ciliary body specimens for the experimental group was significantly lower than that at the previous time point (3.4 ± 0.15%; р< 0.05; Table 1), indicating a reduced microcirculatory bed.
 Discussion
Our findings of a reduction in melatonin production at night are in agreement with the findings of others [7, 22]. In animals maintained under conditions of around-the-clock illumination, marked circulatory abnormalities were observed in the iris and ciliary body at time points until 12 months. Particularly, vessels were markedly dilated and hyperemic, and capillaries of cilary body terminal processes appeared paretically dilated and congested and showed signs of stasis and erythrocyte sludge.
At 12 to 28 months of animal maintenance under conditions of around-the-clock illumination, iris and ciliary body vascular circulatory abnormalities appeared to be changed by sclerotic abnormalities. The sclerotic abnormalities observed in the control animals were interpreted as age related. It should be, however, noted, that, in animals maintained under conditions of around-the-clock illumination, vascular sclerotic changes appeared substantially earlier, and were much more marked, than in control animals. In addition, there was a difference between groups in the type of vessels affected by sclerosis. Thus, in control animals, only small arteries were sclerotic. In animals maintained under conditions of around-the-clock illumination, however, not only small arteries, but also small veins and microcirculatory vessels (i.e., arterioles, capillaries, and venules) and connective tissue substance were sclerotic, and isolated arterioles and capillaries appeared hyalinized.
It may be hypothesized that the circulatory and subsequent sclerotic changes induced in the ciliary body by the conditions of around-the-clock illumination are functionally accompanied by abnormal aqueous production.
It is clear that the changes like newly formed rough bundles of collagen fibers found in an analogue of the Schlemm canal exert a very negative effect on hydrodynamics of the eye in general due to compromised aqueous outflow.
Disclaimer: The authors state that the thoughts expressed in this article are their own and not the official positions of the institutions
Sources of support: none
Conflict of Interest Declaration: the authors declare that there are no real or potential conflicts of interest (financial, personal, professional, or other interests) that could affect the content or conclusions of this manuscript.
References
1.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001 Aug 15;21(16):6405-12.
2.Gooley JJ , Rajaratnam SM, Brainard GC, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010 May 12;2(31):31ra33.
3.Korf HW, Schomerus C, Stehle JH. The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv Anat Embryol Cell Biol. 1998; 146:1-100.
4.Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc. 1983 Aug;42(11):2783-9.
5.Ruby NF, Brennan TJ, Xie X, et al. Role of melanopsin in circadian responses to light. Science. 2002 Dec 13;298(5601):2211-3.
6.Wetterberg L. Light and biological rhythms. J Intern Med. 1994 Jan;235(1):5-19.
7.Bondarenko LA, Gubina-Vakulik GI, Sotnik NN. [Effects of constant light on the rabbit’s circadian melatonin rhythm and pineal gland structures]. Problemy endocrinnoi patologii. 2005;4:38-45. Russian.
8.Gubina-Vakulik GI, Bondarenko LA, Sotnik NN. [Prolonged around-the-clock illumination as a factor of accelerated aging of the pineal gland]. Uspekhi gerontologii. 2007; 20(1): 92-5. Russian.
9.Alkozi HA, Wang X, Perez de Lara M, Pintor J. Presence of melanopsin in human crystalline lens epithelial cells and its role in melatonin synthesis. Exp Eye Res. 2017; 154:168–76.
10.Cahill GM, Besharse JC. Light‐sensitive melatonin synthesis by Xenopus photoreceptors after destruction of the inner retina. Vis Neurosci. 1992; 8: 487–90.
11.Hamm HE, Menaker M. Retinal rhythms in chicks: circadian variation in melatonin and serotonin N‐acetyltransferase activity. Proc Natl Acad Sci USA. 1980 Aug;77(8):4998-5002.
12.Iuvone PM, Tosini G, Pozdeyev N, et al. Circadian clocks, clock networks, arylalkylamine N‐acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005; 2005 Jul;24(4):433-56.
13.Martin XD, Malina HZ, Brennan MC, et al. The ciliary body - the third organ found to synthesize indoleamines in humans. Eur J Ophthalmol. 1992;2:67–72.
14.Pescosolido N, Gatto V, Stefanucci A, Rusciano D. Oral treatment with the melatonin agonist agomelatine lowers the intraocular pressure of glaucoma patients. Ophthalmic Physiol Opt. 2015 Mar;35(2):201-5.
15.Nedzvetskaya OV, Kolot AV, Bondarenko LA. [Investigating retinal morphological changes in experimental hypopinealism]. Oftalmologiiaa. Vostochnaia Evropa. 2015; 2 (25): 35-40. Russian.
16.Matviienko AV, Stepanova LV. [Guidelines on preclinical morphological studies in preclinical medication trials]. Kyiv: State Pharmacological Center at the Ministry of Health of Ukraine; 2001. Ukrainian.
17.Lillie RD. [Histopathologic technic and practical histochemistry]. Moscow: Mir; 1969. Russian.
18.Sarkisov DS, Perova IuL, editors. [Microscopic technique: a manual]. Moscow: Meditsina; 1996. Russian.
19.Lapach SN, Chubenko AV, Babich PN. [Statistical methods in medical and biological studies using Excel]. 2nd ed. Kyiv: Morion; 2007. Russian.
20.Avtandilov G.G. [Basics of quantitative pathological anatomy]. Moscow: Meditsina; 2002. Russian.
21.Sergiienko VI., Bondareva IB. [Mathematical statistics in clinical studies] Moscow: Geotar Meditsina; 2000. Russian.
22.Lemaigre-Voreaux P. Melatonine et Lumiere. Lux. 1986; 139:183-97.
|
