J.ophthalmol.(Ukraine).2022;2:21-26.
|
http://doi.org/10.31288/oftalmolzh202222126 Received: 12 November 2021; Published on-line: 30 April 2022 Improving the ablastic capacity of intravitreal chemotherapy for retinoblastoma N. F. Bobrova, T. A. Sorochynska, S. A. Tronina, O. Iu. Bratishko SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: filatov.detskoe7@gmail.com TO CITE THIS ARTICLE: Bobrova NF, Sorochynska TA, Tronina SA, Bratishko OIu. Improving the ablastic capacity of intravitreal chemotherapy for retinoblastoma. J.ophthalmol.(Ukraine).2022;2:21-6. http://doi.org/10.31288/oftalmolzh202222126 Background: Intravitreally (IV) administered cytostatics are believed to be a promising local chemotherapy for retinoblastoma (RB) because this approach enables the highest level of chemotherapeutic agent after its direct injection into the ocular cavity. Intravitreal administration is, however, invasive. Among the factors that prevent the wide use of intravitreal chemotherapy (IVitC) for RB is the risk of intraocular complications. In addition, exteriorization of the intraocular tumor may occur through the injection channel or extrabulbar tumor spread may occur. Purpose: To develop a technique of IVitC for intraocular RB to improve its ablastic capacity through the prevention of extrabulbar tumor spread. Material and Methods: An ablastic technique of IVitC was developed at the Department of Pediatric Eye Disorders, the Filatov institute, and used to perform 253 IV injections in 30 children (37 eyes) with T1 to T3 retinoblastoma. Results: The advantages of the newly developed IVitC technique are as follows: achieving ocular hypotony without additional paracentesis; preventing reflux from the vitreous cavity by displacing the conjunctiva above the intravitreal entry point and forming an obliquely perpendicular injection channel; treating the site of scleral puncture with cotton swab tamponade and applying antibiotic solution subconjunctivally; preventing an infection of the vitreous and scleral thinning in repeat IVitC. There were no perioperative or postoperative complications. In addition, there were no signs of extrabulbar tumor spread during follow-up after IVitC. The number of IV injections per eye ranged from 1 to 13. Conclusion: An improved ablastic capacity of the developed IVitC technique was achieved by reducing (a) reflux from the vitreous through a number of above manipulations and (b) traumatic effect of intervention, as well as preventing complications, which enabled the minimal invasiveness and safety of the technique. Keywords: retinoblastoma, chemotherapy, intravitreal injection
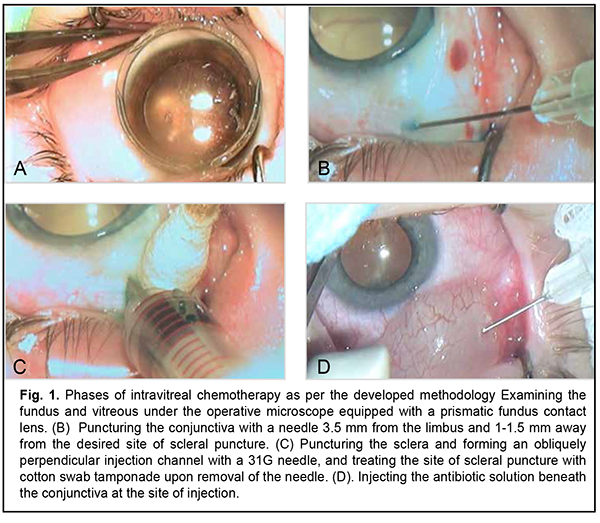
Introduction Some researchers believe that intravitreally (IV) administered cytostatics are a promising local chemotherapy for retinoblastoma (RB) because this approach enables the highest level of chemotherapeutic agent after its direct injection into the ocular cavity, with the concentration in the body kept low [1-9]. Intravitreal administration is, however, invasive. It has been reported that the adverse events associated with intravitreal chemotherapeutic injection for RB include ocular hypertension, cataract, intraocular hemorrhage, endophthalmitis, etc. [10, 11]. In addition, exteriorization of the intraocular tumor may occur through the injection channel [12, 13] or extrabulbar tumor spread may occur, leading to (a) increased risk of orbit tumor spread and metastasis and (b) decreased survival. Consequently, to make intravitreal chemotherapeutic injection as safe as possible, measures should be designed to prevent tumor spread after local chemotherapy for intraocular RB. The purpose of this study was to develop a technique of intravitreal chemotherapy (IVitC) for intraocular retinoblastoma to improve its ablastic capacity through the prevention of extrabulbar tumor spread. Material and Methods An ablastic technique of IVitC was developed at the Department of Pediatric Eye Disorders, the Filatov institute, and used to perform 253 IV injections in 30 children (37 eyes) with T1 to T3 retinoblastoma. A preoperative bilateral eye examination was performed under general anesthesia and included tonometry and anterior segment biomicroscopy using a 30 SL-M slit lamp microscope (Opton, Berlin, Germany. In addition, slit-lamp gonioscopy with a Goldmann lens or microscopic gonioscopy with a 10-mm Volk mini four-mirror goniolens was performed, if required. A dilated posterior segment examination included binocular ophthalmoscopy (using a Keeler Spectra IRIS Spectacle Indirect Ophthalmoscope with a 20 D Volk lens), slit-lamp biomicroscopy (using a Volk Digital Wide Field Lens or Volk three-mirror gonio fundus lens) and wide-field fundus imaging using PanoCam camera system (Visunex Medical Systems, Inc., Fremont, CA). The Aviso Ultrasound Unit (Quantel Medical, Clermont-Ferrand, France) with 10-MHz and 20-MHz probes was used to perform ultrasound scanning of the posterior segment and, if required, the anterior segment. Number of tumor foci; location, topography and size of the tumor focus; thickness of the tumor; presence, type and dissemination of vitreous seeding; and presence of sites with no tumor foci or seeding were assessed. Magnetic resonance imaging (MRI) of the brain and orbit was performed to exclude pineoblastoma and orbital tumor involvement. Follow up duration ranged from 6 months to 2 years. Results We have developed the ablastic IVitC procedure which is performed in the operative room and under the operative microscope, requires general anesthesia and maximum midriasis, and is as follows: First, intramuscular diuretic injection is administered at an age-dependent dose thirty minutes prior to IV chemotherapy injection. Second, the fundus and vitreous are examined under the operative microscope equipped with a prismatic fundus contact lens (Fig. 1a) to select a tumor-free site with no vitreous seeding 3.5-5 mm from the limbus (in case of repeat IV, a site is selected at a meridian different from those of previous injections) for IV injection. Third, the conjunctiva is (a) punctured with a needle approximately 1-1.5 mm away from the desired site of scleral puncture and (b) displaced gently above the intravitreal entry point (Fig. 1b). Fourth, the sclera is punctured with a 31G needle to form an obliquely perpendicular scleral injection channel (Fig. 1c). Fifth, the 0.1-mL cytostatic solution is prepared extempore by dissolving in 0.9% NaCl at the dilution dependent on indication, and, with the position of the needle in the vitreous cavity controlled to avoid lens trauma and contact with the lens, the solution is slowly injected into the vitreous. Sixth, the needle is promptly removed, and the site of injection is treated by tamponade with a cotton swab (Fig. 1c). Seventh, the antibiotic solution is injected beneath the conjunctiva at the site of injection until a bead is formed (Fig. 1d). Eighth, the eye is carefully shaken with a forceps in all directions for 30 to 60 seconds to enable even distribution of the cytostatic in the vitreous. Ninth, sterile dressing is put on the eye and not changed till the next day postoperative review.
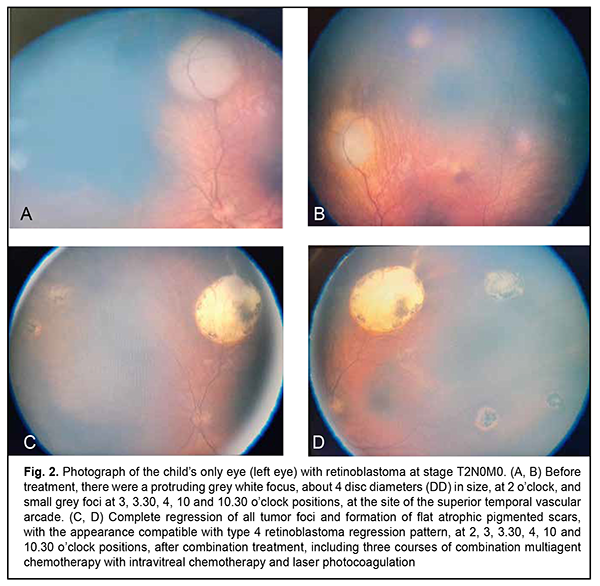
The number of IV injections per eye ranged from 1 to 13. There were no perioperative or postoperative complications. In addition, there were no signs of extrabulbar tumor spread, recurrence in the orbit, or remote metastasis during follow-up after intravitreal chemotherapy. An example case is described below demonstrating the efficacy of the developed IV chemotherapy procedure. Example case A 16-mmonth child underwent surgery for advanced T3 retinoblastoma of the right eye at the Department of Pediatric Eye Disorders, the Filatov institute. The eye was enucleated by high-frequency electric welding (HFEW) using the methodology developed at the Department of Pediatric Eye Disorders [14], and a locomotor stump of the ocular prosthesis was formed using the Ecoflon orbital implant. Histology of the enucleated right globe showed retinoblastoma with no invasion, and the left eye was healthy. At 10 months, examination of the left eye under anesthesia showed multifocal retinoblastoma (six tumor foci varying in size), including a protruding grey white focus, about 4 disc diameters (DD) in size, at 2 o’clock, and small grey foci at 3, 3.30, 4, 10 and 10.30 o’clock positions, at the site of the superior temporal vascular arcade. Teller acuity was 0.13 OS. The intraocular pressure (IOP) was 18 mm Hg OS (Fig. 2a).
The diagnosis was changed to bilateral retinoblastoma, with anophthalmia OD and multifocal retinoblastoma at stage Т2N0M0 OS. The treatment included three courses of combination multiagent chemotherapy using the methodology developed at the department [15]. These included (a) three IVitC with melphalan at a dose of 10 µg with concurrent 3-drug (carboplatin, etoposide, and vincristine (CEV)) chemoreduction and (b) further consolidation therapy in the form of 10 µg intravitreal melphalan and laser photocoagulation of tumor foci using the methodology developed at the department [16]. Totally, the infant received four IVitC at various meridians from 10.30 to 12.30 o’clock positions. The treatment resulted in complete regression of tumor foci and formation of flat atrophic pigmented scars, with the appearance compatible with type 4 RB regression pattern (Fig. 2b). The infant was followed up for 29 months. At present, the disease state is stable and visual acuity is 0.7.
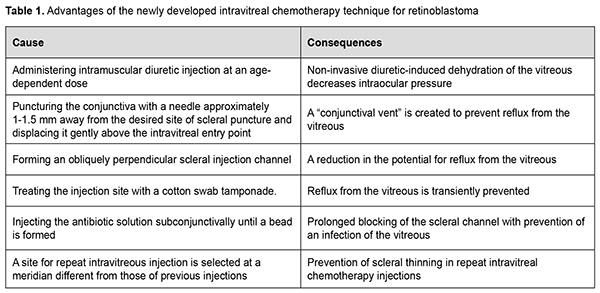
Discussion Best practice for safe IVitC includes a number of measures. Preoperative diagnostic assessment should include meticulous ophthalmoscopic examination and sonographic examination to localize tumor foci, assess dissemination of vitreous seeds, and select a tumor-free site with no vitreous seeding for IV injection [2, 6, 7, 15, 17]. Next, measures should be taken to prevent tumor cell dissemination during IVitC. The IVitC technique for RB developed by Munier and colleagues [13] appears to be the closest to ours and is described briefly below. First, they create a transient hypotony by an anterior chamber paracentesis, aspirating the same volume (0.1-0.15 mL) as the one to be injected into the vitreous. Second, a 32G needle mounted on a tuberculin syringe is introduced perpendicularly 2.5–3.5 mm from the limbus at the desired meridian opposite the seeds through the conjunctiva and sclera under microscope viewing until the needle tip reaches the center of the vitreous cavity. Third, upon removal of the needle three cycles of freeze and thaw cryoapplications are given at the injection site. Finally, the eye is carefully shaken with a forceps in all directions to enable even distribution of the drug in the vitreous. We believe that the IVitC technique of Munier and colleagues has the disadvantages as follows. First, the technique includes additional intraocular manipulation (anterior chamber paracentesis) in an eye with an intraocular tumor. This makes the intravitreal injection technique more difficult due to ocular hypotony and anterior shift of the iridolenticular diaphragm, leading to changes in the intraocular topography. Second, numerous IVitC-related paracenteses contribute to a decrease in anterior chamber depth, formation of anterior synechiae, anterior chamber angle closure, and potential development of secondary glaucoma. Third, introducing the injection needle perpendicularly to the surface of the globe may result in reflux from the vitreous cavity. Fourth, three cycles of freeze and thaw cryoapplications given at the injection site will result in an increase both in the operative time and in postoperative inflammation, creating the conditions for local thinning of the sclera, which is especially relevant to the case of repeated IV injections. Finally, three scleral cryoapplications at the pars plana ciliaris may lead to further ciliary atrophy and progressive progressive hypotony, especially in repeated injections. In the IVitC technique developed by us (Patent of Ukraine No. 144,595 issued 12.10.2020, Bulletin No. 19) [17], ocular hypotony is achieved by preoperative application of intramuscular injection of a diuretic, whereas reflux from the vitreous cavity is prevented by the following measures: displacing the conjunctiva above the intravitreal entry point; forming an obliquely perpendicular injection channel; treating the site of scleral puncture with cotton swab tamponade and applying antibiotic solution subconjunctivally, which also prevents an infection of the vitreous; and selecting different meridians for repeat IVitC to prevent scleral thinning. The above measures enable improving the IVitC technique for RB, advancing prevention of tumor cell dissemination during IVitC, and decreasing the risk of perioperative and postoperative complications after IVitC for RB (Table 1). Therefore, compared with the IVitC technique for RB developed by Munier and colleagues [13], our technique has the following distinctive features: (1) Creating hypotony preoperatively in a non-invasive way; (2) Preventing reflux from the vitreous cavity by: (a) Puncturing the conjunctiva with a needle approximately 1-1.5 mm away from the desired site of scleral puncture and displacing it gently above the intravitreal entry point; (b) Forming an obliquely perpendicular scleral injection channel; (c) Applying cotton swab tamponade of the site of injection upon removing the needle; (d) Injecting the antibiotic solution beneath the conjunctiva at the site of injection until a bead is formed; (3) Decreasing the risk of perioperative and postoperative complications after IVitC for RB due to rejecting the use of additional manipulations (an anterior chamber paracentesis and cryoapplications at the injection site) and selecting a site for repeat IVitC injection at a meridian different from those of previous injections. Conclusion The developed IVitC technique for intraocular RB is minimally invasive, easy to use and prevents perioperative and postoperative complications. High ablastic capacity was achieved by reducing reflux from the vitreous, and confirmed by the absence of extrabulbar tumor spread, tumor recurrence in the orbit, and remote metastasis. Conflict of Interest Statement. The authors declare no conflict of interest. Funding Support. There are no external sources of funding. References 1.Bobrova NF, Naumenko VO, Sorochynska TA, Tronina SA, Dembovetska GM. [Diagnostic assessment and treatment of children with retinoblastoma, a malignant retinal tumor: a protocol]. Oftalmol Zh. 2012;1:80-4. Russian. 2.Bobrova NF, Sorochynska TA. [Combined (intravitreal and intravenous) polychemotherapy in organ-preserving treatment for retinoblastoma. Oftalmol Zh. 2011;2:38-44. Ukrainian. 3.Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol. Acta Ophthalmol (Copenh). 1961;39:569-76. 4.Frederici TJ. Intravitreal injections: AAO's Focal Points. Clinical Modules for Ophthalmologists. 2009;27(8, module 2):1–12. 5.Ghassemi F, Khodabande A. Risk definition and management strategies in retinoblastoma: current perspectives. Clin Ophthalmol. 2015 Jun 8;9:985-94. 6.Seregard S, Kock B, Trampe E,Seregard S. Intravitreal chemotherapy for recurrent retinoblastoma in an only eye. Br J Ophthalmol. 1995 Feb;79(2):194-5. 7.Shields CL, Manjandavida FP, Arepalli S, et al. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319-325. 8.Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013 Oct;97(10):1231-6. 9.Suzuki S, Aihara Y, Fujiwara M, Sano S, Kaneko A. Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol. 2015 May;59(3):164-72. 10.Jager RD, Aiello LP, Patel SC, et al. Risks of intravitreous injection: a comprehensive review. Retina. 2004 Oct;24(5):676-98. 11.Kaneko A, Suzuki S. Eye-Preservation Treatment of Retinoblastoma with Vitreous Seeding. Jpn J Clin Oncol. 2003 Dec;33(12):601-7. 12.Munier FL, Gaillard M-C, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013 Jul;27(3):147-50. 13.Munier FL, Soliman S, Moulin A, et al. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilization of the needle track. Br J Ophthalmol. 2012;96(8):1084–1087. 2012 Aug;96(8):1084-7. 14.Bobrova NF, editor. [Retinoblastoma]. Odesa: Izdatelskii tsentr; 2020. Russian. 15.Bobrova NF, Sorochynska TA. [Method of combination treatment for retinoblastoma]. Patent of Ukraine 55690 issued on December 12, 2010. Ukrainian. 16.Bobrova NF, Naumenko VO, Sorochynska TA, Komarnytska TI. [Method of treatment for residual and recurrent retinoblastomas located posterior to the equator]. Patent of Ukraine UA 133456 issued on March 10, 2018. Bulletin No.7/2018. Ukrainian. 17.Bobrova NF, Sorochynska TA, Bratishko OIu. [Method of intravitreal chemotherapy for retinoblastoma]. Patent of Ukraine UA 144595 issued on October 12, 2020. Bulletin No.19/2020. Ukrainian.
|