J.ophthalmol.(Ukraine).2022;2:15-20.
|
http://doi.org/10.31288/oftalmolzh202221520 Received: 24 November 2021; Published on-line: 30 April 2022 Optical coherence tomography angiography as an indicator of the efficacy of treatment for choroidal neovascularization F. A. Bakhritdinova 1, Z. R. Maksudova 2, N. A. Usmanova 2; F. M. Urmanova 1 1 Tashkent Medical Academy, Tashkent (Uzbekistan) 2 Innovative Clinic DMC; Tashkent (Uzbekistan) TO CITE THIS ARTICLE: Bakhritdinova FA, Maksudova ZR, Usmanova NA, Urmanova FM. Optical coherence tomography angiography as an indicator of the efficacy of treatment for choroidal neovascularization. J.ophthalmol.(Ukraine).2022;2:15-20. http://doi.org/10.31288/oftalmolzh202221520 Background: Choroidal neovascularization (CNV) occurs in as much as 11.3% of patients with pathological myopia, and is a major cause of visual disability associated with irreversible loss of central vision. The advent of optical coherence tomography angiography (OCTA) has opened up new opportunities for objective documentation and real-time qualitative and quantitative evaluation of CNV in the course of therapy. Purpose: To assess the efficacy of anti-vascular epithelium growth factor (VEGF) therapy with ranibizumab in CNV associated with pathological myopia using OCTA. Material and Methods: Thirty seven anti-VEGF-treatment naive patients (37 eyes) with myopic CNV were involved in the study. All study participants received an intravitreal ranibizumab injection (in accordance with the manufacturer’s recommendations) followed by as needed (PRN) retreatment. Results: Complete subretinal fluid resorption with adherence of the neurosensory retina was observed in all the 37 eyes; the mean number of intravitreal ranibizumab injections required was 4.56 ± 0.1. Over the 18-month follow up period, best-corrected visual acuity (BCVA) improved from 0.12 ± 0.03 to 0.42 ± 0.04 in 27 eyes (72.97%). OCTA patterns of CNV activity tended to fade, with the characteristic presence of isolated long filamentous vessels having a “dead tree” appearance. Conclusion: Application of OCTA, an information-rich modality, in pathological myopia, facilitates a personalized approach to determining the need for anti-VEGF therapy and selecting the mode of administration of anti-VEGF agents based on the CNV activity. Keywords: choroidal neovascularization, anti-VEGF therapy, optical coherence tomography angiography, pathological myopia
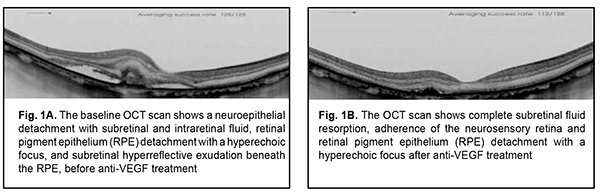
Introduction Myopia is a serious public health problem in many countries. Of all the students graduating from secondary schools in the Central Asia, 70% to 80% are affected by myopia, and of these myopes, 20% to 30% have pathological myopia [1-6]. Choroidal neovascularization (CNV) occurs in 5.2-11.3% of patients with pathological myopia [2-8], and is a major cause of visual disability associated with irreversible loss of central vision, because if untreated, myopic CNV is accompanied by a significant damage to photoreceptors and irreversible loss of central vision [1, 3, 9-11]. Issues about conservative and laser treatment for myopic CNV have been discussed by domestic and foreign researchers for years [4, 9, 11-12]. Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy has become the standard-of-care and the recommended first-line treatment option for myopic CNV, and the efficacy of this treatment with retreatment as needed (PRN) has been confirmed by RADIANCE and REPAIR multicenter clinical trials [5, 6]. The efficacy of treatment for myopic CNV in randomized multicenter clinical trials was assessed by changes in best-corrected visual acuity (BCVA) and optical coherence tomography (OCT)-based changes in neurosensory retinal morphology [5, 6, 11]. Fluorescein angiography has always been considered the gold standard for the diagnosis, classification and activity monitoring of CNV, but its frequent application is limited by potential side effects associated with the invasiveness of the procedure [1, 7-9, 12]. OCT angiography has provided a novel imaging modality as it allowed for dyeless imaging of choroidal neovascularization. OCTA is a safe and non-invasive modality with no side effects, which makes it promising for documentation and real-time qualitative and quantitative evaluation of CNV as well as CNV monitoring in the course of therapy [1- 4, 7, 10, 11]. Although there have been numerous studies on myopic CNV, specific signs of CNV activity and morphological changes in CNV in the presence of anti-VEGF therapy are still relevant [5, 6, 9-11], which makes the current study important. The purpose of this study was to assess the efficacy of ranibizumab therapy in CNV associated with pathological myopia using OCTA. Material and Methods Thirty seven patients (37 eyes; age, 19 to 47 years; mean age, 32 ± 3.8 years) who presented with anti-VEGF-treatment naive myopic CNV were involved in the study. In all cases, CNV was initially identified at presentation. At baseline, best-corrected visual acuity (BCVA) ranged from 0.08 to 0.45, with a mean value of 0.12 ± 0.03. The pre-treatment myopic refractive error ranged from –4.0 D –16.5 D (mean value, –8.5 ± 4.8 D), and the anteroposterior length, from 26.0 mm to 30.50 mm (mean value, 27.3 ± 2.0 mm). To assess the efficacy of anti-VEGF therapy, patients received a standard eye examination, including BCVA, ocular biomicroscopy with Goldmann and Meinster lenses (Ocular Instruments, Bellevue, WA), and swept-source OCT and OCTA (DRI OCT Triton; Topcon, Tokyo, Japan). The DRI OCT Triton features a 1-µm, 1,050-nm light source with a scanning speed of 100,000 A-scans/second. At each visit, 3.0 x 3.0 mm OCTA scans of the retina were obtained to assess choroidal structural changes in myopic CNV after treatment with time. CNV membrane area (mm2) was calculated using standard software. Morphological signs of CNV activity were assessed by the method developed by Coscas [13, 14]. The OCTA data were used to determine the location and type of CNV. The signs of CNV exudation were identified using the guidelines issued by an international group of retinal experts [5, 6]. All study participants received an intravitreal ranibizumab (Novartis Pharma AG) 0.5 mg/0.05 ml injection (in accordance with the manufacturer’s recommendations) followed by as needed (PRN) retreatment until complete suppression of CNV activity as evidenced by OCT or OCTA. This was a single-center, prospective clinical study. The study protocol was approved by a local Ethical Committee and written informed consent was obtained from all participants. Statistical analysis was performed using SPSS 19.02 and Microsoft Excel 2017 software. Neovascular process was monitored with OCTA at day 30 after each injection. Follow-up duration was 18 months. Results Prior to treatment, all patients complained of reduced vision and metamorphopsia. The study group included only patients with OCT and OCT evidence of active macular neovascularization. Of all the affected eyes, 56.75% (21 eyes) had subfoveal CNV; 34.23% (12 eyes), juxtafoveal CNV; and 10.81% (4 eyes), extrafoveal CNV. Edema involved the fovea in all affected eyes. All patients showed OCT signs of CNV exudation including subretinal fluid in 81.08% (30 eyes), intraretinal cystic cavities in 75.67% (28 eyes), intraretinal hemorrhage in 37.83% (14 eyes), and subretinal hyperreflective exudation in 83.78% (31 eyes) (Fig. 1а).
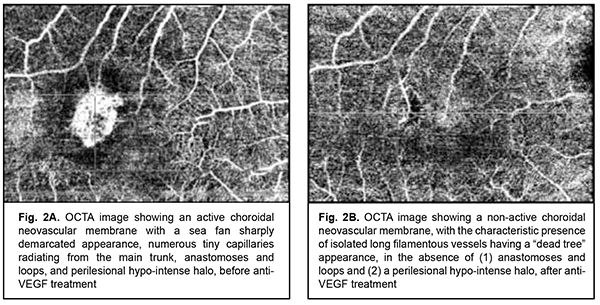
The mean central retinal thickness was 247 ± 13.96 µm (range, 189 µm to 358 µm). The mean retinal thickness in the area of CNV was 339 ± 10.26 µm (range, 267 µm to 421 µm). All patients showed OCTA patterns of CNV activity like a neovascular membrane with a typical lacy or sea fan sharply demarcated appearance; branching networks of numerous tiny capillaries radiating from the main trunk; and anastomoses and loops. There were well-seen peripheral arcades with hypointense halo around CNV (Fig. 2a).
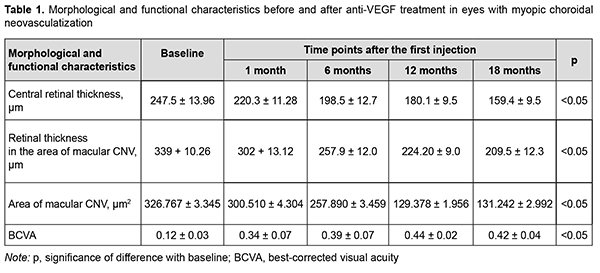
At day 30 after the first intravitreal ranibizumab injection, an improvement in vision and anatomic measures was noted in 27 eyes (72.97%). In addition, an improvement in exudative signs was noted in 26 eyes (70.27%), and no change in exudative signs, in 11 eyes (29.73%). OCTA patterns of CNV activity tended to fade, with the characteristic presence of isolated long filamentous vessels having a “dead tree” appearance, in the absence of (1) anastomoses and loops and (2) a perilesional hypo-intense halo. However, there were still mild signs of patterns of CNV activity like peripheral loops and isolated anastomoses in 81.08% of cases (30 eyes), which required another intravitreal injection. Therefore, a decision on whether to retreat or not was based on whether OCTA-based angiographic signs of CNV activity were marked. Each affected eye required 2 to 7 ranibizumab injections to suppress CNV activity. Particularly, 4 eyes (10.81%) required 2 injections; 7 eyes (18.92%), 3 injections; 7 eyes (18.92%), 4 injections; 10 eyes (27.02%), 5 injections; 5 eyes (13.51%), 6 injections; and 4 eyes (10.81%), 7 injections. The mean number of intravitreal ranibizumab injections required was 4.56 ± 0.1. Table 1 shows changes in morphological and functional characteristics after anti-VEGF therapy for myopic CNV.
Over the 18-month follow up period, BCVA improved from 0.12 ± 0.03 to 0.42 ± 0.04 in 27 eyes (72.97%), did not improve in 8 eyes (21.62%); and did not change in 2 eyes (5.40%). In addition, OCT-derived central retinal thickness reduced from 247 ± 13.96 µm to 159.40 ± 9.50 µm in 35 eyes (94.59%), and did not change in 2 eyes (5.40%). Moreover, OCT-derived retinal thickness in the area of CNV reduced from 339 ± 10.26 µm to 209.50 ± 12.30 µm, whereas OCTA-derived CNV area reduced from 326.767 ± 3.345 µm2 to 131.242 ± 2.992 µm2 in 34 eyes (91.89%), and did not change in 3 eyes (8.10%). However, patterns of CNV activity were absent in 36 eyes (97.29 %), and present in one eye (2.7 %). It is noteworthy that in no case did we observe an increase in CNV area or signs of CNV exudation in the neurosensory retina after anti-VEGF therapy over the 18-month follow up period. In all the patients, suppression of CNV activity was accompanied by an improvement in BCVA and complete disappearance of OCT-derived signs of CNV exudation in the neurosensory retina. Other findings included complete adherence of the neurosensory retina, incomplete adherence of the retinal pigment epithelium, resorption of intraretinal cysts, and absorption of intraretinal and subretinal hemorrhage. The thickness of subretinal hyperreflective exudation decreased, and the margins become clear (Fig. 1B). There was a reduction in OCTA-derived patterns of CNV activity, and the appearance of macular CNV changed to that of a “dead tree” (Fig. 2B). By the end of the follow-up period, the visual and anatomic outcome of anti-VEGF therapy was still good in most (94.59%) patients, but BCVA reduced due to progressive subretinal fibrosis in spite of the absence of signs of activity of the pathological process in 5.4% of patients. Discussion The results of the current study demonstrated that OCTA is an imaging modality that provides information-rich vascular images in monitoring anti-VEGF therapy for CNV in pathological myopia. According to an international group of experts, anti-VEGF therapy is indicated for active CNV which is confirmed by the following signs: increased retinal thickness, due to fluid accumulation within or beneath the retinal layers (the neurosensory retina or RPE), as evidenced by OCT images; intraretinal or subretinal hemorrhage; leakage as evidenced by fluorescein angiography [1-3, 11, 12]. Currently, OCTA is the gold standard for evaluating the activity of CNV. A limitation of the current study is that, unlike fluorescein angiography, OCTA does not allow determining the state of the vascular wall and leakage [11, 12]. However, during fluorescein angiography, vessels of the fundus are filled with dye in a step-by-step manner, and, unlike OCTA, a complete image of vascular disease cannot be provided without any delay. OCTA does not require dye, and, consequently, a patient will have no dye-associated side effects [1, 2, 12]. Both OCTA and fluorescein angiography are limited with regard to the potential for identifying early signs of CNV activity. The introduction of OCTA has expanded the opportunities for diagnostic evaluation and treatment of CNV. The analysis of OCTA data enabled to identify the patterns of CNV activity. The most informative morphological signs were a well-defined CNV lesion (tortuous lacy-wheel or sea-fan shaped); numerous tiny tortuous capillaries; the presence of anastomoses and loops; the presence of a peripheral arcade, and the presence of a perilesional hypo-intense halo. This is in agreement with the findings of others who used the above OCTA-derived patterns of CNV activity for determining the need for retreatment with anti-VEGF therapy 5, 6, 8, 10, 11, 13]. The advance of OCTA has opened up new opportunities for objective documentation and real-time qualitative and quantitative evaluation of CNV as well as CNV monitoring during treatment. The identification of the above patterns of CNV activity allowed to determine the most appropriate data for performing anti-VEGF therapy as needed and to stabilize the neovascular process while preserving visual functions. The anti-VEGF therapy contributed to complete subretinal fluid resorption and adherence of the neurosensory retina with restoration of a normal macular profile and an improvement in mean BCVA from 0.12 ± 0.03 до 0.42 ± 0.04. Conclusion First, OCTA is an imaging modality that provides information-rich vascular images in monitoring morphological and morphometric changes in the macular area and changes in CNV activity following anti-VEGF therapy in myopic CNV. Second, application of OCTA, an information-rich modality, in pathological myopia, (1) facilitates a personalized approach to determining the need for anti-VEGF therapy and selecting the mode of administration of anti-VEGF agents based on the CNV activity and (2) enables assessing adequately the efficacy of the selected management option.
References 1.Chan WM, Ohji M, Lai TYY, Tano Y, Lam DSC. Choroidal neovascularization in pathological myopia: an update in management. Br J Ophth. 2005 Nov;89(11):1522-8. 2.Min CH , Al-Qattan HM, Lee JY, Kim J-G. Macular Microvasculature in High Myopia without Pathologic Changes: An Optical Coherence Tomography Angiography Study. Korean J Ophthalmol. 2020 Apr; 34(2): 106–12. 3.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010 Aug;117(8):1595-611, 1611.e1-4. 4.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016 May;123(5):1036-42. 5.Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12 month results from the REPAIR study. Ophthalmology. 2013 Sep;120(9):1944-5.e1. 6.Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014 Mar;121(3):682-92.e2. 7.Neelam K, Cheung CM, Ohno-Matsui K, et al. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012 Sep;31(5):495-525. 8.Raecker ME, Park D.-W, Lauer AK. Diagnosis and treatment of CNV in myopic macular degeneration. Eye Net Magazine. 2015: 35–7. 9.Wong TY, Ohno-Matsui K, Leveziel N, et al. Myopic choroidal neovascularization: current concepts and update on clinical management. Br J Ophthalmol. 2015 Mar;99(3):289-96. 10.Ng WY, Ting DS, Agrawal R, et al. Choroidal structural changes in myopic choroidal neovascularization after treatment with Antivascular endothelial growth factor over 1 year. Invest Ophthalmol Vis Sci. 2016 Sep 1;57(11):4933-9. 11.Wang Y, Hu Z, Zhu T, et al. Optical Coherence Tomography Angiography-Based Quantitative Assessment of Morphologic Changes in Active Myopic Choroidal Neovascularization During Anti-vascular Endothelial Growth Factor Therapy. Front Med (Lausanne). 2021 May 7;8:657772. 12.Venediktova OA, Saksonov SG, Suk SA. [Diagnostic value of optical coherence tomography and fluorescein angiography in the evaluation of the dynamics of regression of classical subretinal neovascular membranes at high-complicated myopia after combined use of ranibizumab and transpupillary thermotherapy]. Ukrainian Scientific Medical Youth Journal. 2012; 2:53 55. Russian. 13.Coscas G, Lupidi M, Coscas F, Français C, Cagini C, Souied EH. Optical coherence tomography angiography during follow-up: qualitative and quantitative analysis of mixed type I and II choroidal neovascularization after vascular endothelial growth factor trap therapy. Оphthalmic Res. 2015;54(2):57-63. 14.Coscas GJ, Lupidi M, Coscas F, Cagini F, Souied EH. OCTA versus traditional multimodal imaging in assessing the activity of exudative AMD. A new diagnostic challenge. Retina. 2015 Nov;35(11):2219-28.
Conflict of Interest. The authors declare that there are no conflicts of interest that would affect their opinion of the subject matter or materials discussed in this manuscript.
|