J.ophthalmol.(Ukraine).2022;2:54-56.
|
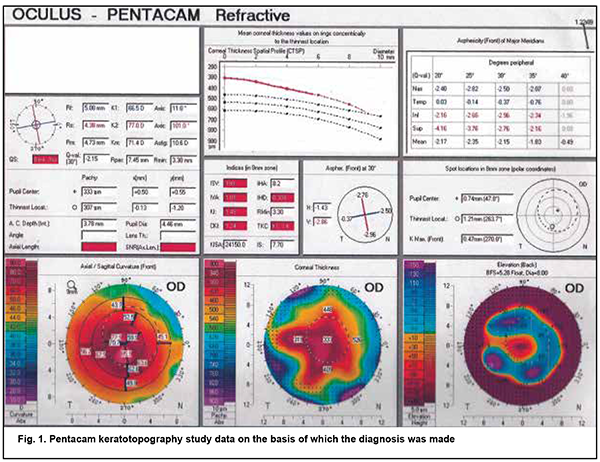
http://doi.org/10.31288/oftalmolzh202225456 Received: 06 Janurary 2022; Published on-line: 30 April 2022 Performing DALK complicated by Descemet's membrane macroperforation in keratoconus without conversion to penetrating keratoplasty: a case report V. N. Serdiuk ¹, N. G. Klopotskaya ¹, S. B. Ustimenko ², Ye. N. Maidenko ², Ye. B. Storozhenko ², V. V. Tikhomirova ² 1 Dnipro State Medical University; Dnipro (Ukraine) 2 Dnipropetrovsk Regional Clinical Ophthalmological Hospital; Dnipro (Ukraine) TO CITE THIS ARTICLE: Serdiuk VN., Klopotskaya NG., Ustimenko SB., Maidenko YeN, Storozhenko YeB, Tikhomirova VV. Performing DALK complicated by Descemet's membrane macroperforation in keratoconus without conversion to penetrating keratoplasty: a case report. J.ophthalmol.(Ukraine).2022;2:54-6. http://doi.org/10.31288/oftalmolzh202225456 We report a case of successful deep anterior lamellar keratoplasty (DALK) for kearatoconus in the presence of intraoperative Descemet's membrane (DM) macroperforation in a 10-year boy. Intraoperative optical coherence tomography–guided femtosecond laser-assisted DM separation from the periphery to the center facilitated keeping the anterior chamber angle open and avoiding a subsequent increase in intraocular pressure. In addition, the size of the graft was made 0.4 mm larger that the size of the trephination hole, which enabled fitting of the graft margins and trephination margins, whereas precise deep femtosecond laser corneal dissection enabled (a) preventing a shock wave effect from the laser pulses and (b) DM adherence to the stroma of the graft. At 5 months after surgery, uncorrected visual acuity (UCVA) was 0.5; keratometry values OD were R1, 7.67 mm; К1, 44.0 D; R2, 7.30 mm; К2, 46.2 D; Rm, 7.48 mm; Km, 45.1 D; and Astig, 2.2 D. Anterior chamber angle was 41.4º. The DM adhered well to the donor stroma, the DM defect was practically not visualized on imaging, and endothelial cell density was 2719 cells/mm2 in the operated eye. At 7 months after surgery, the patient’s UCVA was 0.6 OD and corrected visual acuity with a spherical equivalent of +3.0 D was 0.9. Keywords: deep anterior lamellar keratoplasty, Descemet's membrane macroperforation Keratoconus is a non-infectious degenerative condition with lesions of the corneal stromal collagen matrix in which the normally round and regular shape of the cornea progressively thins and bulges until it is a cone-like, irregular shape. The reported incidence varies from 1:2,000 to 1:500,000 [3]. Corneal transplantation (or keratoplasty) is still the only treatment for late-stage keratoconus. Deep Anterior Lamellar Keratoplasty (DALK) is a widely recognized treatment for keratoconus, and makes it possible (a) to avoid the complications (e.g., postoperative glaucoma) characteristic of penetrating keratoplasty (PK) [3, 4, 5] and (b) to preserve patient’s endothelial cells. The endothelial cell loss (ECL) is more intensive during 6 months after DALK (10%), and remains almost constant thereafter until month 12, whereas PK patients exhibit an ECL of 33-43% over 12 months. However, DALK is a more difficult procedure to perform than PK, and intraoperative Descemet's membrane (DM) tear (microperforation or macroperforation) can occur with DALK, requiring conversion to PK. The rate of conversion from DALK to PKP due to loss of the integrity of DM varies from 2.9% to 23% and depends on the surgeon’s experience, ability to successfully bare the DM, and indication for keratoplasty [3, 5]. The case becomes even more complicated when a large DM tear occurs at an early stage of stromal dissection, before complete baring of DM or before reaching a satisfactory pre-descemetic plane. Dissecting the remaining thick residual stromal layer will apply more traction forces on the edges of the tear making the conversion to PKP very likely [1]. The management of DM perforation includes intracameral air tamponade, stromal patching, fibrin glue and suturing of the defect [1, 2, 5]. Most DALK surgeons are now in agreement that DALK can still be successfully completed with almost all microperforations (less than one quarter of the cornea) using air/gas tamponade for DM apposition to the donor cornea. There is no, however, agreement on whether DALK can still be successfully completed with macroperforations. Some authors believe that in cases where the surgeon encounters macroperforations (greater than one quadrant of the cornea), it may not be easy to complete the dissection of the posterior lamella fully up to the Descemet's or pre-Descemet's region, and, in such cases, better visual and refractive outcomes are achieved by converting the procedure to a full thickness PK [5]. Others [1, 4], however, have reported on eyes with large DM tears in which DALK was successfully completed. A boy of 10 years diagnosed with grade 4 keratoconus OD was under our care. In autumn of 2020, he was found to have reduced vision. The family history was significant for an elder brother with keratoconus. The boy was born from the fourth pregnancy and was the fourth child of his mother. He had no concurrent general medical condition. The diagnosis was based on the results of keratotopography study of the right eye using Pentacam (Oculus, Wetzlar, Germany) (Fig. 1). Prior to surgery, uncorrected visual acuity (UCVA) was 0.02 OD; intraocular pressure (IOP) as assessed by pneumotonometry, 14 mmHg; and corneal thickness OD as assessed by keratopachymetry, 293 µm. In addition, the eye was quiet and exhibited Haab's striae and the cornea thin and cone-shaped.
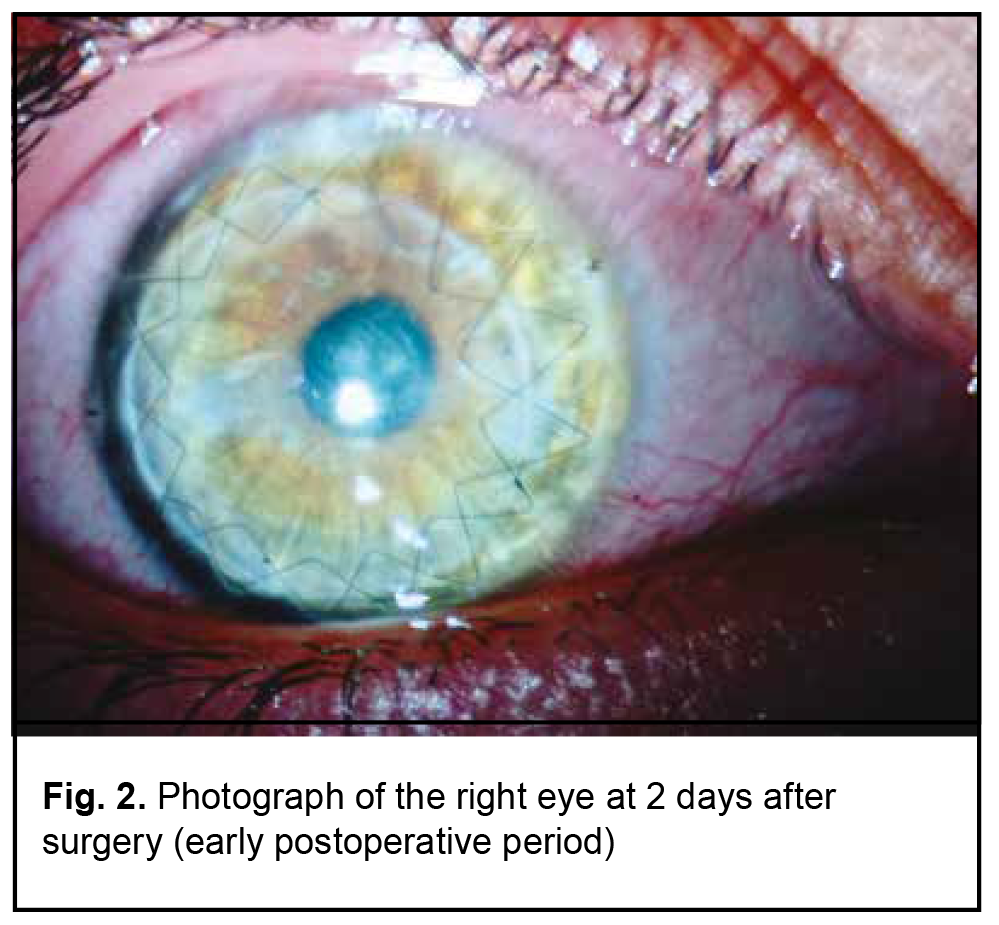
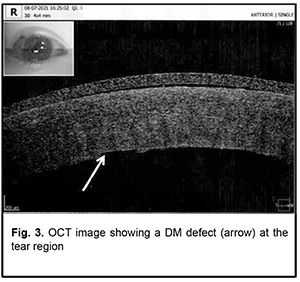
On July 15, 2021, the boy received femtosecond laser-assisted DALK surgery with the use of keratobioimplant. Intraoperative optical coherence tomography (OCT)–guided Victus® femtosecond laser (Bausch & Lomb)–assisted corneal dissection was performed using the following laser settings: spot spacing, 2 µm; energy, 1.8 nJ per pulse for the receipient and 1.9 nJ per pulse for the donor; side cut angles, 90° for the receipient and 92-97° for the donor; pulse duration, 600 to 800 femtoseconds. The size of the graft was made 0.4 mm larger that the size of the trephination hole (anterior and posterior diameters of the cornea were 8.4 mm and 8.0 mm, respectively, for the recipient, and 8.8 mm and 8.1 mm, respectively, for the donor). Therefore, the profile of the graft cut facilitated placing the graft into the recipient bed like a watch glass. The AE-4210 forceps were used to strip the Descemet membrane from the donor cornea. The surgical microscope (HS Hi-R NEO 900A NIR, Haag-Streit Surgical GmbH & Co. KG, Wedel, Germany) with an intraoperative OCT (iOCT) system was used intraoperatively, which enabled to assess the trephination depth; visually monitor the insertion of the 27-gauge air injection cannula (Fogla 27G Air injection cannula, Storz Ophthalmics, USA) using the Big Bubble technique; assess the residual stromal thickness; and detect any residual space in the graft-host interface at the end of DALK surgery. During injection of a gas bubble into the anterior chamber, due to marked corneal thinning and corneal cicatrization, there was a gas pressure-induced linear rupture in the Descemet's membrane along the optical zone, practically along an entire diameter of the cornea (7.9 mm), but there was no collapse of the anterior chamber. Because the anterior chamber was still stable, the surgeon continued performing careful iOCT-guided separation of the stroma from the DM from periphery to the center, while trying to minimize tractions at the margins of the tear. This approach made it possible to bare the entire Descemet's membrane and to form the trephination bed with a correct angle between the stroma and DM, without the excessive stromal tissue, which, in turn, allowed preventing a shock wave effect from the laser pulses. The keratobioimplant was placed on the recipient cornea and sutured with two running stitches. A 10% perfluoropropane gas mixture was introduced into the anterior chamber at the end of DALK surgery. Postoperatively, the patient received systemic immunosuppressive/ anti-inflammatory therapy with corticosteroids, topical antibacterial therapy with fluoroquinolones, and reparative therapy for 2 weeks on the in-patient basis. In addition, the anti-inflammatory and reparative therapy was continued on the out-patient basis for 4 months after he was discharged home. At 2 days after surgery, UCVA was 0.1 OD, the right eye appeared irritated, and the graft was transparent. Fig. 2 shows the affected eye at 2 days after surgery (early postoperative period). The OCT image showed a DM defect at the tear region (Fig. 3).
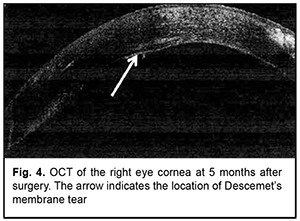
At 3 months after surgery, UCVA was 0.3 OD; sphere and cylinder values from autorefraction were +1.25 D and -4.0 D, respectively, OD. Keratometry values were R1, 7.46 mm; К1, 45.3 D; R2, 7.14 mm; К2, 47.3 D; Rm, 7.30 mm; and Km, 46.2 D. Central corneal thickness was 537 mm; anterior chamber angle was 40.9̊. At 5 months after surgery, the patient had UCVA 0.5 OD with the eye quiet and keratobioimplant epithelialized and sutured with two running stitches; the anterior chamber was moderately shallow and the optical media were clear. IOP as assessed by pneumotonometry was 10 mmHg OD and 12 mmHg OS. Keratometry values OD were R1, 7.67 mm; К1, 44.0 D; R2, 7.30 mm; К2, 46.2 D; Rm, 7.48 mm; Km, 45.1 D; and Astig 2.2 D. Anterior chamber angle was 41.4º. Central corneal thickness was 572 mm; minimal thickness, 516 mm; anterior chamber depth, 3.06 mm. Ultrasound biomicroscopy and OCT of the anterior segment demonstrated that the Descemet membrane adhered well to the donor stroma, and the DM defect was practically not visualized on imaging (Fig. 4). Endothelial cell density was 2719 cells/mm2 in the operated eye (OD) and 2763 cells/mm2 in the non-operated eye (OS).
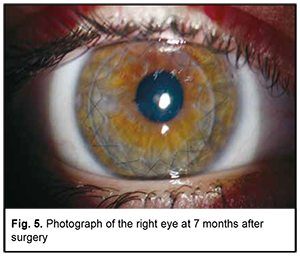
At 7 months after surgery, the patient’s UCVA was 0.6 OD and corrected visual acuity with a spherical equivalent of +3.0 D was 0.9. Fig. 5 shows right eye in the late period (7 months) after surgery.
This case demonstrates that, even in cases with a large DM tear, iOCT–guided femtosecond laser–assisted DALK makes it possible to continue a preliminary planned course of surgical intervention without converting to PK. In addition, femtosecond laser–assisted DM separation from the periphery to the center facilitated keeping the anterior chamber angle open and avoiding a subsequent increase in IOP. Moreover, the size of the graft was made 0.4 mm larger that the size of the trephination hole, which enabled fitting of the graft margins and trephination margins, whereas precise deep femtosecond laser corneal dissection enabled (a) preventing a shock wave effect from the laser pulses and (b) DM adherence to the stroma of the graft. Finally, even in cases with macroperforation of the DM, DALK surgery enables obtaining a high endothelial cell density and high visual acuity, and minimizing corneal astigmatism in the late period after surgery. Disclaimers. The opinions expressed in this article are those of the authors and not the official positions of the institution or foundation. Source(s) of support: none Conflict of Interest. The authors declare that there are no conflicts of interest that would influence their opinion on the subject matter or materials described and discussed in this manuscript.
References 1.Hashem AN, Tolees SS, Samir A. Novel technique for the management of macroperforation during deep anterior lamellar keratoplasty. Clin Ophthalm. 2019 Oct 23;13:2075-2080. 2.Huang OS, Htoon HM, Chan AM, et al. Incidence and outcomes of intraoperative Descemet membrane perforations during deep anterior lamellar keratoplasty. Am J Ophthalmol. 2019 Mar;199:9-18. 3.Janiszewska-Bil D, Czarnota-Nowakowska B, Krysik K, et al. Comparison of long-term outcomes of the lamellar and penetrating keratoplasty approaches in patients with keratoconus. J Clin Med. 2021 May 29;10(11):2421. 4.Kodavoor SK, Deb B, Ramamurthy D. Outcome of deep anterior lamellar keratoplasty patients with intraoperative Descemet’s membrane perforation: A retrospective cross-sectional study. Ind J Ophthalm. 2018 Nov;66(11):1574-1579. 5.Nanavaty MA, Vijjan KS, Yvon C. Deep anterior lamellar keratoplasty: a surgeon's guide. J Current Ophthalmol. 2018 Jul 10;30(4):297-310.
|