J.ophthalmol.(Ukraine).2021;6:25-30.
|
http://doi.org/10.31288/oftalmolzh202162530 Received: 02 November 2021; Published on-line: 21 December 2021 Ultrastructural features of epiretinal membranes after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy Vira S. Ponomarchuk, N.I. Molchaniuk, M. M. Umanets SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: v.zavodnaya@gmail.com TO CITE THIS ARTICLE: Ponomarchuk Vira S, Molchaniuk NI, Umanets MM. Ultrastructural features of epiretinal membranes after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy. J.ophthalmol.(Ukraine). 2021;6:25-30. http://doi.org/10.31288/oftalmolzh202162530 Background: Proliferative diabetic retinopathy (PDR) can cause a loss of vision due to complications like vitreous hemorrhage and tractional retinal detachment, which require vitrectomy. The source of bleeding is usually in pathologic new blood vessels in the fibrovascular epiretinal membrane (ERM). Anti-vascular endothelial growth factor agents are widely used for prevention of intraoperative and postoperative hemorrhagic complications in vitrectomy for PDR. Purpose: To examine ultrastructural features of ERM after intravitreal injection of various doses of aflibercept in patients with PDR. Material and Methods: Five patients (5 eyes) were included in the study. ERM specimens with neovascular vessels were taken intraoperatively from the five patients for histological microscopy studies. Specimen No. 1 was taken from the ERM (control ERM) of the patient who did not receive preoperative injection of aflibercept. In addition, specimen No. 2 was taken on day 3 after intravitreal injection of 1.0 mg aflibercept; specimen No. 3, on day 5 after intravitreal injection of 1.0 mg aflibercept; specimen No. 4, on day 3 after intravitreal injection of 2.0 mg aflibercept; and specimen No. 5, on day 5 after intravitreal injection of 2.0 mg aflibercept. Results: We found that aflibercept had a positive effect on obliteration of newly formed vessels and capillaries. There was a decrease in the size of the lumen of ERM microvessels with an increase in the injected dose and time after injection. In addition, endothelial cells in the newly formed vessels and fibroblasts exhibited active protein synthesis metabolism on day 3 after intravitreal injection of 1.0 mg aflibercept or 2.0 mg aflibercept. Keywords: proliferative diabetic retinopathy, epiretinal membrane, aflibercept
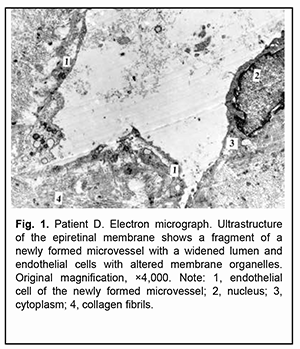
Introduction Proliferative diabetic retinopathy (PDR) can cause a steady loss of vision due to complications like vitreous hemorrhage and tractional retinal detachment (TRD), which require vitrectomy [1]. The development of PDR is characterized by pathological vasculogenesis. Vasculogenesis, unlike angiogenesis, is a process by which new vessels are generated from endothelial progenitors via differentiation [2]. Intraoperative and postoperative vitreous hemorrhage is the most common complication of vitrectomy for PDR [3]. The source of bleeding is usually in pathologic new blood vessels in the fibrovascular epiretinal membrane (ERM) which become damaged during membrane excision [4]. Anti-vascular endothelial growth factor (VEGF) agents are widely used for prevention of intraoperative and postoperative hemorrhagic complications in vitrectomy for PDR [5]. Previous studies have demonstrated a positive effect of anti-VEGF agents in combination with vitrectomy in the treatment of patients with PDR [6,7,8]. Aflibercept is a selective anti-VEGF agent which is widely used in eye care practice. It binds not only to all isoforms of VEGF-A, but also to VEGF-B and placental growth factor [9]. We have previously demonstrated that pretreatment with 1.0-mg or 2.0-mg intravitreal aflibercept resulted in vitrectomy for PDR resulted in fibrosis of the epiretinal fibrovascular membrane with a marked proliferative component and obliteration of newly formed blood vessels as early as day 3 or day 5 after injection [10]. To the best of our knowledge, there have been few reports on the studies of the ultrastructure of newly formed vessels and epiretinal membranes in patients with PDR. Kohno and colleagues [11] examined the histopathologic effect of a single intravitreal injection of bevacizumab (1.25 mg/eye) on newly formed vessels in eyes with PDR, and concluded, that intravitreal injection of bevacizumab may induce changes in immature, newly formed vessels of PDR tissue, leading to endothelial apoptosis with vascular regression, while inducing normalization of premature vessels by increasing pericyte coverage and reducing vessel fenestration. Given that both bevacizumab and aflibercept are anti-VEGF agents, it is reasonable to assume that aflibercept also may have an effect on fenestration of endothelial cells. Dumbrova and colleagues [12] assessed the ultrastructural changes in the rabbit’s choroid and retina after injections of selective and non-selective anti-VEGF agents, and concluded that 0.025-mg injection of ranibizumab caused the mildest ultrastructural changes in the choroid and retina. Vidinova and Vidinov [13] examined the ultrastructural changes in the subretinal neovascular membranes occurring after the intravitreal administration of bevacizumab in patients with age-related macular degeneration (AMD). Subretinal membranes in AMD patients consisted mainly of fibroblasts, isolated or grouped RPE cells and elements of blood [13]. In another study [14], these authors tried to point out the ultrastructural differences between proliferative tissues in PDR patients with and without vitreous haemorrhage in regard to the clinical practice and proper timing of vitreo-retinal surgery. The results pointed out that blood in the vitreous cavity alters the ultrastructure of the existing PDR proliferations in respect to making them more dense and prone to contractions. To the best of our knowledge, there has been no report on the study of ultrastructural changes in ERM in patients with PDR after injection of aflibercept. Therefore, the purpose of this study was to examine ultrastructural features of ERM after intravitreal injection of various doses of aflibercept in patients with PDR. Material and Methods This was an open-label, prospective interventional study. The study protocol was approved by the Bioethics Committee of the Institute of Eye Disease and Tissue Therapy, NAMS of Ukraine (Protocol no. 1 issued 15.10.2018). Before treatment, informed consent was signed by all patients, and they were educated about the possible consequences and complications of the surgical procedure. Five patients (5 eyes) aged 31 to 58 years with a neovascular glial form of PDR and the presence of fibrovascular epiretinal membrane with a marked proliferative component were included in the study [15]. A PDR was complicated by TRD with involvement of the macular region in 3 eyes, and combined tractional and rhegmatogenous retinal detachment (TRRD), in 2 eyes. Patients underwent an eye examination which included visual acuity assessment, refractometry, tonometry, static automated perimetry, biomicroscopy, gonioscopy and ophthalmoscopy. Patients with a history of vitrectomy, prior anti-VEGF injections, or the presence of uveitis (or intraocular inflammation), iris rubeosis, elevated intraocular pressure (IOP), total vitreous hemorrhage, central retinal vein or branch occlusion, central retinal artery occlusion or ERM with a moderate proliferative component were excluded. Baseline visual acuity ranged from 0.01 to 0.04, and baseline intraocular pressure (IOP), from 18.0 mm Hg to 23.0 mmHg. Initial complicated cataract was observed in 2 eyes and 3 eyes had an intraocular lens (IOL) implanted. Intravitreal injection was administered in the operating room after epibulbar anesthesia using a standard protocol. The tip of a 29-gauge needle was inserted in the inferior inner quadrant of the eye, and aflibercept was injected through the pars plana ciliaris into the vitreous body under indirect ophthalmoscopy guidance. Each patient underwent a 25G three-port vitrectomy using an Alcon Constellation 25-G vitrectomy machine (Alcon Laboratories, Inc., Fort Worth, TX, USA) and OMS-800 OFFISS microscope (Topcon, Tokyo, Japan) on day 3 to day 5 after intravitreal aflibercept injection. Core and peripheral vitrectomy and posterior hyaloid peeling and excision over 3600 were performed using a wide angle viewing system BIOM system (Oculus, Wetzlar, Germany). Epiretinal membranes were removed completely. Epiretinal membrane specimens with neovascular vessels were taken intraoperatively from the five patients for histological microscopy studies. Specimen No. 1 was taken from the ERM (control ERM) of the patient who did not receive preoperative injection of aflibercept. In addition, specimen No. 2 was taken on day 3 after intravitreal injection of 1.0 mg aflibercept; specimen No. 3, on day 5 after intravitreal injection of 1.0 mg aflibercept; specimen No. 4, on day 3 after intravitreal injection of 2.0 mg aflibercept; and specimen No. 5, on day 5 after intravitreal injection of 2.0 mg aflibercept. Epiretinal membrane fragments were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4), post-fixed with 1% osmium tetroxide in phosphate buffer (pH 7.4), dehydrated through an ascending ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with lead citrate according to the procedure described by Reynolds [16], and observed with a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine). Results The control ERM consisted of differently directed short and thin collagen fibrils that were either normal or showed destruction. Single cells with altered organelles and newly formed microvessels were seen amongst fibrils. These microvessels varied in the width of the lumen, and some of them displayed a significantly widened lumen (Fig. 1).
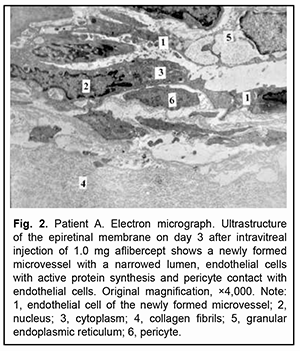
Large microvessels were irregularly shaped, and their endothelial cells had a large nucleus surrounded by a narrow cytoplasm. The microvessel wall was covered mostly by thinned hyaloplasm of endothelial cells with electron-dense matrix and single altered organelles. Some of these cells exhibited clusters of lysosomes and osmiophilic granules. Endothelial cells in other microvessels appeared necrotic. Only new capillaries were fenestrated, and they showed few fenestrations. Endothelial cell basal membrane appeared confluent with the plasmalemma of these cells which appeared thicker and electron denser than the plasmalemma at the luminal side of these cells. In some newly formed vessels, the basal membrane was not visualized. Pericytes were rarely seen; they were large and had a large nucleus surrounded by a narrow cytoplasm. Their structures could not be differentiated from each other. Some of these vascular cells appeared necrotic. The ERM fragment that had been taken on day 3 after intravitreal injection of 1.0 mg aflibercept showed active proliferation compared to that taken before injection: the connective tissue showed an increased number of (a) collagen fibrils which were arranged more closely to each other and (b) the cells (especially large fibroblasts) with signs of activated protein synthesis. Newly formed blood microvessels were seen among collagen fibrils, and the lumen of these vessels was narrower compared to that in the control ERM. The microvessel wall was covered only by large endothelial cell bodies (hypertrophied cells), and the number of organelles in the hyaloplasm of these cells was larger than that in the control ERM. These cells had active metabolism (particularly, metabolism of protein synthesis), which was indicated by an enlarged (and sometimes lobular) nucleus with invaginations or deep folds; an active nucleolus; large extended cisternae of the granular endoplasmic reticulum (GER) which were in contact with karyoplasms; and numerous hyaloplasm mitochondria. Microclasmatosis was observed at the luminal side of microvessels. Of note is that, in newly formed microvessels of these ERMs, the lumen was practically half as narrow as that in microvessels of the ERM in controls. Fenestrations were not visualized in the images (Fig. 2).
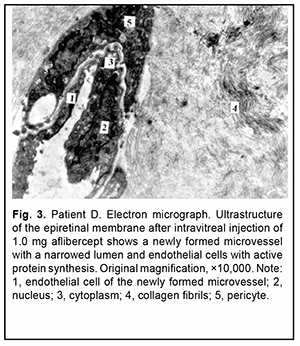
The ERM fragment that had been taken on day 5 after intravitreal injection of 1.0 mg aflibercept showed active synthesis in some fibrobasts and destruction in some other fibrobasts. Large ERM sites appeared empty due to destruction of collagen fibrils and breakup of individual cells. There were few isolated newly formed microvessels in the ERM, and they were either obliterated or had a lumen that appeared like a narrow stripe. Endothelial cells of these vessels showed destruction and necrosis, and in this case no fenestrations were observed (Fig. 3).
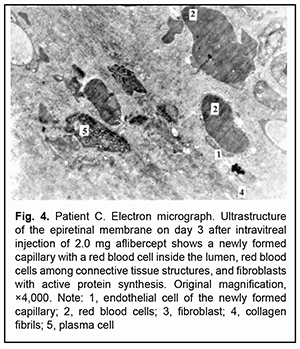
On day 3 after intravitreal injection of 2.0 mg aflibercept, some newly formed microvessels with pericytes and capillaries were seen in the ERM, and they (especially newly formed capillaries) were mostly obliterated. Their endothelial cells appeared collapsed. Red blood cells were seen in a narrow lumen of some of newly formed microvessels, and a portion of these cells appeared deformed. In addition, red blood cells and numerous cells of the vitreous and connective tissue with signs of active synthesis were seen amidst closely arranged collagen fibrils. There were single microvessels with a somewhat widened lumen which, however, was substantially narrower compared to controls, and this lumen contained deformed red blood cells. Of note is that these microvessels had no fenestrations and their endothelial cells appeared active. Moreover, some destruction of collagen fibrils and connective tissue cells was characteristic for these ERMs (Fig. 4).
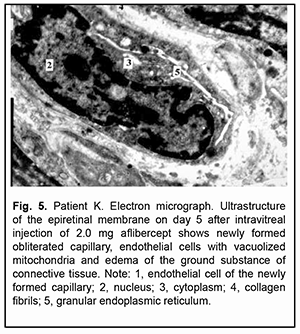
On day 5 after intravitreal injection of 2.0 mg aflibercept, newly formed microvessels in ERM tissue were completely obliterated. Endothelial cells of some microvessels had an almost normal ultrastuctural appearance, whereas those of other microvessels appeared degenerated and showed ultrastructural disruption. Clumps of obliterated newly formed microvessels without fenestrations were seen at some locations. Connective tissue cells were seen amongst the vessels, and some of these cells had signs of activated protein synthesis, whereas others appeared disrupted. Despite an active process of collagen fibril synthesis, substantial ERM sites appeared empty or collagen fibrils showed fibrin necrosis (Fig. 5).
Discussion The analysis of the study results demonstrated that the control ERM showed newly formed microvessels with lumens of various sizes. Their endothelial cells either had an almost normal ultrastructural appearance or showed disruption of organelles. Fenestrated endothelial cells were seen only in the capillaries. Short and thin collagen fibrils were mostly scattered and chaotically arranged amongst cells of the vitreous and connective tissue in the ERM. On day 3 after intravitreal injection of 1.0 mg aflibercept, the ERM showed proliferation of fibroblasts with signs of activated protein synthesis (these signs were also exhibited by some endothelial cells of newly formed microvessels), and an increased number of collagen fibrils compared to the control ERM. In addition, the lumen in the newly formed microvessels was substantially narrower than that in the newly formed microvessels of the control group. On day 5 after intravitreal injection of 1.0 mg aflibercept, the ERM showed mostly destructive changes in all its structural components, with a decreased number of newly formed microvessels which were either obliterated or had a lumen that appeared like a narrow and electron-lucent stripe. Their endothelial cells showed destruction or necrosis. On day 3 after intravitreal injection of 2.0 mg aflibercept, the number of newly formed vessels present in the ERM was low, and they were mostly obliterated. The microvessel wall consisted of endothelial cells showing destruction and single pericytes. However, collagen fibrils of the connective tissue were arranged more closely to each other and in an organized pattern in the ERM (which indicates their strength), and single red blood cells were seen amongst them. Vidinova and Vidinov [14] tried to point out the ultrastructural differences between proliferative tissues in PDR patients with and without vitreous hemorrhage, and examined the materials with microscopy and histochemically. In the proliferations of patients with vitreous hemorrhage, macrophages and erythrocytes were more commonly detected. In the tissue capillaries of young new vessels prevailed, with only a very thin layer of endothelial cells comprising their walls, which is in agreement with our findings. In the extracellular matrix among the proteoglycan complexes, Vidinova and Vidinov [14] found lots of residual elements of blood. They concluded that blood in the vitreous cavity alters the ultrastructure of the existing PDR proliferations in respect to making them more rigid and prone to contractions. On day 5 after intravitreal injection of 2.0 mg aflibercept, newly formed vessels in the ERM were completely obliterated; some of their endothelial cells appeared degenerated and showed ultrastructural disruption; and collagen fibrils were necrotic. Thus, in the use of intravitreal injection of 1.0 mg aflibercept, the size of the lumen of ERM microvessels appeared to decrease with an increase in the time from injection to vitrectomy, whereas complete obliteration of newly formed ERM microvessels was observed practically on day 3 after intravitreal injection of 2.0 mg aflibercept. This study demonstrated that intravitreal injection of 1.0 mg aflibercept or 2.0 mg aflibercept not only facilitated the narrowing of newly formed vessels, but also activated protein synthesis both in connective tissue cells (i.e., fibroblasts) and endothelial cells of newly formed microvessels on day 3. Although a reduction in these phenomena in the ERM was observed on day 5, an increase in destruction occurred in the ERM, which resulted in a decrease in ERM density and thus created a potential for easier removal of the membrane. It is noteworthy that single pericytes were found in newly formed vessels, but not in newly formed capillaries. Fenestrations in the thinned sites of endothelial cell hyaloplasma were found only in newly formed capillaries, and these were single fenestrations. This is in agreement with Kohno and colleagues [11] who found that a single intravitreal injection of bevacizumab may induce changes in immature, newly formed vessels of PDR tissue, particularly, by reducing vessel fenestration. In addition, Vidinova and Vidinov [13] examined the effect of bevacizumab on the ultrastructure of choroidal neovascular membranes in patients with age-related macular degeneration (AMD) and also demonstrated that intravitreally administered bevacizumab causes changes in the new vessel walls and in the extracellular matrix components, which diminishes the fenestration of the endothelial cells. Moreover, others [17-19] demonstrated penetration of bevacizumab through the blood retinal barrier after intravitreal injection in the monkey, with subsequent morphological changes in the choriocapillaris. They noted a reduction in the fenestration in the choriocapillaris as early as 24 hours after intravitreal injection of bevacizumab [17-19]. Therefore, we found that intravitreal injection of aflibercept resulted in the narrowing and obliteration of newly formed vessels depending on the injected dose and time after injection. A longitudinal reduction in the fenestration in endothelial cells of newly formed capillaries prevents leakage of plasma substances into the perichoroidal space. In sum, the development of destruction processes in the ERM and obliteration of newly formed vessels after intravitreal injection of aflibercept prevented vitreous hemorrhage and created a potential for easier removal of the membrane.
References 1.Shkvorchenko DO, Levina LV. [Retinotomy and retinectomy in the management of proliferative diabetic retinopathy complicated by anterior proliferative vitreoretinopathy]. In: [Proceedings of the National Russian Conference on Modern Technologies for the Treatment of Vitreoretinal Pathology]. Moscow; 2006. p. 205-10. Russian. 2.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008 Apr 30;41(4):278-86. 3.Hendrick AM, Gibson MV, Kulshresthra A. Diabetic Retinopathy. Prim Care. 2015 Sep;42(3):451-64. 4.Sawa H, Ikeda T, Matsumoto Y. Neovascularization from scleral wound as cause of vitreous rebleeding after vitrectomy for proliferative diabetic retinopathy. Jpn J Ophthalmol. Mar – Apr 2000;44(2):154-60. 5.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. Feb-Mar 2005;25(2):111-8. 6.Aleman I, Castillo Velazquez J, Rush SW. Ziv-aflibercept versus bevacizumab administration prior to diabetic vitrectomy: a randomised and controlled trial. Br J Ophthalmol. 2019 Dec;103(12):1740-6. 7.Velazquez JC, Aleman I, Rush SW. Bevacizumab before Diabetic Vitrectomy: A Clinical Trial Assessing 3 Dosing Amounts. Ophthalmol Retina. 2018 Oct; 2(10):1010-20. 8.Antoszyk AN, Glassman AR, Beaulieu WT, et al. Effect of Intravitreous Aflibercept vs Vitrectomy With Panretinal Photocoagulation on Visual Acuity in Patients With Vitreous Hemorrhage From Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2020 Dec 15;324(23):2383-95. 9.Abd Elhamid AH, Mohamed AAEA., Khattab AM. Intravitreal Aflibercept injection with panretinal photocoagulation versus early vitrectomy for diabetic vitreous hemorrhage: randomized clinical trial. BMC Ophthalmol. 2020 Apr 6;20(1):130. 10.Ponomarchuk Vira S, Vit VV, Umanets MM. Morphological and ophthalmoscopic features of epiretinal membranes after intravitreal injection of various doses of aflibercept in patients with proliferative diabetic retinopathy. J Ophthalmol (Ukraine). 2021;5:3-9. 11.Kohno R, Hata Y, Mochisuki Y, et al. Histopathology of neovascular tissue from eyes with proliferative diabetic retinopathy after intravitreal bevacizumab injection. Am J Ophthalmol. 2010 Aug;150(2):223-229.e1. 12.Dumbrova NE, Korol AR, Nasinnik IO, et al. [Structural changes in the choroid and retina of the rabbit after intravitreal injections of selective and non-selective anti-VEGF agents]. Oftalmol Zh. 2012;3:68-72. Russian. 13.Vidinova C, Vidinov N. [The effect of bevacizumab on the ultrastructure of choroidal neovascular membranes in patients with age-related macular degeneration (AMD)]. Klin Monbl Augenheilkd. 2009 Jun;226(6):491-5. 14.Vidinova C, Vidinov N. [Comparative ultrastructural analysis of the proliferative tissue in PDR patients with or without vitreous haemorrhage: from ultrastructural approach to clinical practice]. Klin Monbl Augenheilkd. 2007 Mar;224(3):185-9. 15.Ponomarchuk Vira S, Velychko LM, Umanets MM. Vitreous VEGF levels among patients with proliferative diabetic retinopathy depending on the general clinical status and ocular status. J Ophthalmol (Ukraine). 2021;4:19-25. 16.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17(1):208-12. 17.Julien S, Heiduschka P, Hofmeister S, Schraermeyer U. Immunohistochemical localisation of intravitreally injected bevacizumab at the posterior pole of the primate eye: implication for the treatment of retinal vein occlusion. Br J Ophthalmol. 2008 Oct;92(10):1424-8. 18.Heiduschka P, Fietz H, Hofmeister S, et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007 Jun;48(6):2814-23. 19.Peters S, Heiduschka P, Julien S, et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007 Jun;143(6):995-1002.
Author Contributions: Vira S. Ponomarchuk: conducting intravitreal injections, data collection and analysis, writing the text; N. I. Molchaniuk: electron microscopic studies; M.M.Umanets: concept and design of the study, surgical interventions, and editing. Disclosures:The authors declare no conflict of interest. There are no external sources of funding.
|