J.ophthalmol.(Ukraine).2021;6:64-68.
|
http://doi.org/10.31288/oftalmolzh202166468 Received: 28 June 2021; Published on-line: 21 December 2021 A case of persistent recurrent herpes zoster ophthalmicus in a patient with primary mannose binding lectin deficiency D. V. Maltsev Institute of Experimental and Clinical Medicine, Bogomolets National Medical University; Kyiv (Ukraine) E-mail: dmaltsev@ukr.net TO CITE THIS ARTICLE: Maltsev DV. A case of persistent recurrent herpes zoster ophthalmicus in a patient with primary mannose binding lectin deficiency. J.ophthalmol.(Ukraine). 2021;6:64-68. http://doi.org/10.31288/oftalmolzh202166468
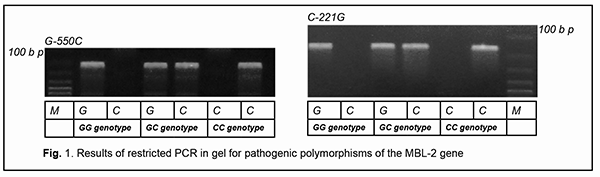
In the current paper, we present an example case of persistent recurrent herpes zoster ophthalmicus with the development of chronic keratoconjunctivitis, corneal opacity, secondary eczema herpeticum and resistance both to antiviral drugs and to anti-allergic medications in a female patient with primary MBL deficiency. Particularly, we demonstrate how the elucidation of the causative immune deficiency and the related administration of specific replacement immunotherapy has become the cornerstone of the achievement of clinical success in the difficult-to-treat patient. Immunological tests demonstrated that the patient had a significantly reduced MBL level (167 ng/ml compared to a norm of 450 ng/ml), whereas other studied immune status characteristics where within normal range. We identified two pathological polymorphisms in the promoter and structural region of MBL-2 (particularly, –550 G/C and 221 C/G), which indicated that the patient had a primary MBL deficiency. It was the fact that the patient had the above genetic immunodeficiency disease that an atypical and unfavorable course of varicella zoster virus infection was associated in this case. The identification of primary MBL deficiency opened the door for new ways of therapy for the difficult-to-treat infectious disease through the specific replacement immunotherapy with the aim of improving immune control of the opportunistic agent through compensation of primary immunodeficiency. Keywords: immunodiagnostics, immunotherapy, keratoconjunctivitis, corneal opacity, immunodeficiency
Introduction Lesions in the eye caused by infection with varicella zoster virus (VZV) are a rather common and usually well treatable ocular pathology [1]. Some forms of recurrent ocular herpes are, however, difficult to treat and they may have unfavorable clinical consequences. VZV reactivation from the trigeminal ganglion can cause ocular lesions with the development of acute keratoconjunctivitis. When reactivation occurs, virus spreads along nerve fibers of the first division of the trigeminal nerve, the ramus ophthalmicus, which innervates the ocular surface [2]. Recurrent VZV-induced keratoconjunctivitis is an important issue in the field of ophthalmology, as the disease frequently results in loss of vision due to opacity of the affected cornea, facilitates the induction of autoimmune complications and requires surgery. Such patients frequently develop the resistance to antiherpetic drugs, which calls for an individual approach to treatment. VZV is an opportunistic pathogen that undergoes reactivation from latency primarily in individuals that are immune-compromised [1, 2]. The identification of primary or secondary immunodeficiency which facilitated reactivation of the virus from latency may provide valuable information regarding the selection of the immunotherapy which, when combined with antiviral drugs, may improve treatment outcomes. Primary mannose binding lectin (MBL) deficiency is a genetic immunodeficiency disease whose clinical manifestations and diagnostic evaluation should be known by doctors of any specialty. This immune dysfunction is present in around 5 to 10 percent of the population [3]. MBL is a pattern-recognition receptor of the innate immune system which can interact with excess mannose on the surface of malignized cells, microorganisms and helminths. The above acute-phase protein is important for its role in the implementation of humoral mechanisms of innate immunity, particularly, neutralization, opsonization, and initiation of the lectin activation pathway of the complement system [4]. In addition, this factor facilitates triggering an antigen-specific immune response, thus facilitating antigen presentation by macrophages and dendritic cells. Clinical manifestations of MBL deficiency have a wide range and include a recurrent and/or abnormally severe course of bacterial [5], viral [6], and herpetic infections [7], parasitic invasions [8], autoimmune [9], allergic [10] and neoplastic syndromes [11], and somatic [12] and obstetrical [13] pathological processes. Reactivated herpes virus infection, sometimes with an atypical course and resistance to recommended treatment, is a typical clinical manifestation of primary MBL deficiency in humans. There have been reports on associations of genetic MBL deficiency with severe infections induced by herpes simplex virus (HSV)-1 [14] and -2 [15], Epstein-Barr virus [16], cytomegalovirus [17], HSV-6 and -7 [4], and HSV-6 [6], but, to the best of my knowledge, not VZV. In the current paper, we present an example case of persistent recurrent herpes zoster ophthalmicus with the development of chronic keratoconjunctivitis, secondary eczema herpeticum and resistance both to antiviral drugs and to anti-allergic medications in a female patient with primary MBL deficiency. Particularly, we demonstrate how the elucidation of the causative immune deficiency and the related administration of specific replacement immunotherapy has become the cornerstone of the achievement of clinical success in persistent recurrent herpes zoster ophthalmicus in a patient with primary MBL deficiency. Case report A female patient, born in 1966, presented with complaints of loss of vision and pain in the right eye and a recurrent right-sided facial rash along the course of the first branch and some part of the second branch of the trigeminal nerve. Initially, a cluster of papulovesicular exanthema, considered typical for common herpes zoster, was observed, which disappeared after a seven-day course of acyclovir. The diagnosis was confirmed by the detection of VZV-IgM antibodies in the patient’s serum. On ocular examination, there were hyperemia and vesicular rash on the skin of the upper eyelid and on the periorbital skin; mixed palpebral and bulbar conjunctival injection, and severe mucous discharge in the conjunctival fornices. In addition, biomicroscopy found numerous coin-shaped opacities on the corneal stromal surface. Moreover, the aqueous humor in the anterior chamber was transparent, pupillary reaction to light normal, and there was no ophthalmoscopic evidence of pathological changes. However, the rash recurred each time shortly after antiviral drug withdrawal. VZV DNA was detected by PCR in saliva and tear samples. After the third recurrence, edema, reddening and itching appeared at the site of facial skin lesions, and, in this connection, a complication in the form of eczema herpeticum was diagnosed by a dermatologist. Exanthema healing time increased, acyclovir became less effective, and the patient required at least a 14-day course of acyclovir. After the sixth recurrence, the rash became chronic with no remission and unresponsive to acyclovir, valacyclovir and famciclovir. Visual acuity in the right eye decreased abruptly. The patient was examined by an ophthalmologist and was diagnosed with chronic herpetic epithelial keratoconjunctivitis. Findings of another ocular examination after three months of therapy included the first signs of keratitis with the first signs of right corneal opacity. Treatment with topical acyclovir and ganciclovir did not result in a reduction in ocular signs of herpetic infection. In addition, there was a gradual reduction in the efficacy of both topical and systemic therapy with corticosteroids administered for eczema herpeticum. Therefore, the patient had several courses of combination therapy which did not result in a complete removal of clinical symptoms. Persistent exacerbation was noted after each withdrawal of therapeutic treatment. In spite of therapeutic interventions, there were signs of disease progression. The first course of combination therapy included: ● Oral acyclovir 800 mg, five times a day for 14 days ● Oral tiloron 125 mg, once daily for 3 days followed by administration of that dose every 48 hours for 5 more days, and ● 3% acyclovir ophthalmic ointment, a small amount (10 mm) of which was applied into the lower conjunctival sac 5 times daily, with subsequent doses separated by approximately 4 hr, for 14 days. The first course of combination therapy resulted in some improvement in amount of herpetic rash and some reduction in corneal and conjunctival inflammation with fast recurrence after withdrawal. The second course of combination therapy included: ● Oral valacyclovir 500 mg, three times daily for a month ● Intramuscular recombinant human interferon alpha-2b, 3 mln units at bedtime on alternate days for 15 days ● Oral methylprednisolone 16 mg, in the morning daily, which was tapered over a month (for relief of eczema herpeticum) ● 3% acyclovir ophthalmic ointment, a small amount (10 mm) of which was applied into the lower conjunctival sac 5 times daily, with subsequent doses separated by approximately 4 hr, for 30 days, and ● 0.1% triamcinolone acetonide ointment, which was applied to the facial skin affected by eczema twice a day for a month. The second course of combination therapy resulted in significant improvement in amount of herpetic rash and significant reduction in corneal and conjunctival inflammation without a complete removal of the symptoms, and with fast recurrence after withdrawal. The third course of combination therapy included: ● Oral famciclovir 500 mg, three times daily for three months ● Intramuscular cycloferon, 2 ml once daily for 3 days followed by administration of that dose at bedtime on alternate days for a total of 10 doses (20 days) and administration of that dose at bedtime once in three days for a total of 20 doses (2 months) ● Intramuscular 10% normal human immunoglobulin, 1.5 ml once in three days for a total of 10 doses ● Oral methylprednisolone 12 mg, once daily in the morning before breakfast for the first month, 8 mg once daily in the morning before breakfast for the second month, and 4 mg once daily in the morning before breakfast for the third month ● Topical ganciclovir ophthalmic gel, five times daily for a month, and twice daily for 2-3 months after improvement, ● Topical betamethasone ointment, twice a day applied to the facial skin affected by eczema twice a day for three months. The third course of combination therapy resulted in significant temporary improvement in amount of herpetic rash and reduction in corneal and conjunctival inflammation without a complete removal of the symptoms, and with fast recurrence after withdrawal. Five months after early clinical symptoms the patient was referred to the clinical immunologist for evaluation of her immune status due to an unfavorable disease course, resistance to recommended therapy and persistent recurrences.. Indeed, a severe, atypical and complicated course of opportunistic infection with signs of resistance to recommended treatment is a well-established clinical indication for an immunological evaluation, because in these cases there are reasonable grounds to suspect an immunocompromised status. Immunological evaluation included complete blood cell count and lymphocyte subpopulation analysis by flow cytometry on a Beckman Coulter Epics XL flow cytometer (Beckman Coulter, Miami, Fla.) and indirect immunofluorescence with monoclonal antibodies to CD markers with two or three immunofluorescent labels (CD3+, CD3+CD4+, CD3+CD8+, CD3–CD19+, CD3–CD16+CD56+, CD3+CD16+CD56+) (reagents from Beckman Coulter). Phagocytosis was assessed using the latex test; phagocytic number (the number of pathogenic units engulfed by one phagocyte), phagocytic index (percentage of phagocytes involved in phagocytosis), number of active phagocytes and phagocytic capacity of blood (the number of pathogenic units that can be neutralized by the phagocytes contained in 1 liter of blood) were determined. Serum levels of IgM, IgG and IgA were determined by the radial immunodiffusion method of Mancini, whereas those of IgE, IgD, IgG1, IgG2, IgG3 and IgG4, using enzyme immunoassay (Vector Best, RF). Myeloperoxidase activity of neutrophils was assessed using enzyme immunoassay at the Neuroimmunology Laboratory of the Romodanov Neurosurgery Institute. The Nitro Blue Tetrazolium assay was performed at the Zabolotnyi Institute of Microbiology and Virology as well at the Neuroimmunology Laboratory, Romodanov Neurosurgery Institute, NAMS of Ukraine. Serum level of mannose binding lectin was determined by ELISA in Germany with assistance of Dr. Rödger Laboratory (Kyiv, Ukraine). Lymphocytosis and monocytosis were detected based on complete blood cell count. Immunological tests demonstrated that the patient had a significantly reduced MBL level (167 ng/ml compared to a norm of 450 ng/ml), whereas other studied immune status characteristics were within normal range. The patient was found to have selective MBL deficiency. Genetic PCR tests were conducted at the Neurobiochemistry Department, Institute of Neurosurgery, to identify the origin of the deficiency. We identified two pathologic polymorphisms in the promoter and structural region of MBL-2 (particularly, –550 G/C and 221 C/G) (Fig. 1), which indicated that the patient had a primary MBL deficiency. It was the fact that the patient had the above genetic immunodeficiency disease that an atypical and unfavorable course of VZV infection was associated in this case. The identification of primary MBL deficiency opened the door for new ways of therapy for the difficult-to-treat infectious disease through the specific replacement immunotherapy with the aim of improving immune control of the opportunistic agent through compensation of primary immunodeficiency.
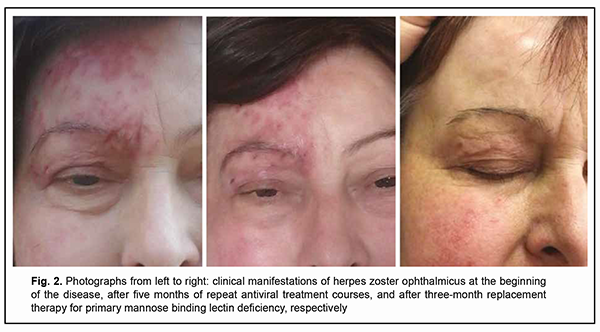
Discussion The pioneer experience in immunotherapy for a patient with a primary MBL deficiency consisted in the use of fresh frozen human plasma for the relief of recurrent erythema multiforme associated with reactivation of herpes simplex virus; the patient’s erythematous eruptions could be controlled with infusions of fresh frozen plasma containing MBL, but not with plasma lacking MBL [18]. There have been further case reports on treatment of MBL deficiency with purified MBL obtained from plasma of healthy donors, which was followed by a dramatic clinical improvement [18, 19]. Although the results of several clinical trials have confirmed that patients with a clinically apparent MBL deficiency benefit from therapy with substitution of the innate immune protein MBL, these products prepared from plasma are still unavailable on the market [20]. Later on, a phase I placebo-controlled double-blinded study [21] was performed in MBL-deficient healthy male subjects to assess the safety, tolerability, and pharmacokinetics of recombinant human MBL (rhMBL). Therefore, in patients with a primary MBL deficiency who developed difficult-to-treat immune-dependent manifestations of this deficiency, replacement therapy with fresh frozen human plasma or cryopreserved human plasma is the only basic treatment strategy available. A recent controlled non-randomized study has demonstrated that drop-wise intravenous human cryopreserved plasma of the appropriate blood group at a dose of 10 ml/kg applied twice a month for 3 months compensated for primary deficiency of MBL, restored the serum lectin pool, and improved treatment outcomes of immunocompromised patients with chronic reactivated herpes virus infections [22]. It is for this reason that human cryopreserved plasma at a dose of 10 ml/kg was applied twice a month for 3 months as an adjunct to conventional valacyclovir therapy in our patient with primary deficiency of MBL. The targeted adjunct immunotherapy facilitated the restoration of the serum level of MBL (with the achievement of a serum MBL level of 570 ng/ml) as early as completion of the first month of treatment. There was a progressive improvement in clinical manifestations of not only herpes zoster but also eczema herpeticum, with an almost complete removal of disease symptoms by the end of the third month of treatment (Fig. 2). It is noteworthy that there was no recurrent herpetic rash or signs of right corneal lesion progression after withdrawal of the combination therapy. The 12-month follow-up after completion of the course of immunotherapy demonstrated that a complete remission of the infectious disease was achieved without the requirement of valacyclovir or repeat courses of replacement immunotherapy for MBL deficiency.
The value of this report is that it vividly demonstrates the obvious benefit of well-planned immunodiagnostics and immunotherapy in severe, atypical forms of opportunistic infections, particularly those with ocular involvement. Indeed, the administration of cryopreserved plasma was a turning point in the clinical management of our patient, and would be impossible without elucidation of the diagnosis of primary MBL deficiency. There have been previous reports on an abnormally frequent recurrence of infections caused by alpha herpes viruses in a primary deficiency of MBL, particularly, on a relapsing-remitting facial palsy and ipsilateral brachial plexopathy caused by HSV-1 in a patient with an abnormally low serum level of MBL [14]. Tang and colleagues [15] reported on a case of HSV-2-associated recurrent aseptic lymphocytic meningitis (Mollaret's meningitis). There is evidence of susceptibility to the development of topical allergic complications in recurrent alpha herpes virus infections in the presence of MBL deficiency. Particularly, Valdimarsson [18] reported on the fast development of erythema multiforme at the site or recurrent exanthema caused by infection with HSV-1 in a child with primary MBL deficiency [18]. However, because, to the best of our knowledge, there have been no reports of reactivation of VZV in primary MBL deficiency in humans, we believe that this is the first report in the world of this association. Valdimarsson have already reported on the experience of using replacement therapy in the management of patients with MBL deficiency and on the capacity of the product prepared from plasma to not only arrest the recurrence of HSV-1 infection, but also to relieve the manifestations of allergic skin reaction associated with this infection [18]. In the current report, we provide evidence of a similar double beneficial effect of replacement immunotherapy in recurrent VZV infection in the presence of primary deficiency of MBL. We have already reported on the benefit of immunodiagnostics and immunotherapy in severe ocular lesions associated with opportunistic infections. Particularly, we have demonstrated the efficacy of using recombinant human gamma interferon in recurrent Toxoplasma chorioretinitis in a patient with primary myeloperoxidase deficiency [23]. In addition, we have reported on the elimination of reactivated HHV-7 and suppression of associated ANA-positive uveitis in a patient with primary MBL deficiency after administration of replacement therapy with cryopreserved human plasma [9]. A wider involvement of clinical immunologists and more advanced and well-planned immunological studies are warranted in the management of ocular patients with severe recurrent and/or atypical opportunistic infections of the eye. References 1.Denier M, Gabison E, Sahyoun M, et al. Stromal Keratitis After Varicella in Children. Cornea. 2020 Jun;39(6):680-4. 2.Nofal A, Fawzy MM, Sharaf El Deen SM, El-Hawary EE. Herpes zoster ophthalmicus in COVID-19 patients. Int J Dermatol. 2020 Dec;59(12):1545-1546. 3.Maltsev DV. [Mannose binding lectin deficiency]. Ukrainskyi terapevtychnyi zhurnal. 2015; 1:80-9. Ukrainian. 4.Maltsev DV. [Cases of mannose binding lectin deficiency]. Ukrainskyi medychnyi chasopys. 2015;2 (106):122-7. Ukrainian. 5.Gao DN, Zhang Y, Ren YB, et al. Relationship of Serum Mannose-Binding Lectin Levels with the Development of Sepsis: a Meta-analysis. Inflammation. 2015 Feb;38(1):338-47. 6.de Morais VMS, de Lima ELS, Cahú GG, et al. MBL2 gene polymorphisms in HHV-8 infection in people living with HIV/AIDS. Retrovirology. 2018 Nov 27;15(1):75. 7.Lambourne J, Agranoff D, Herbrecht R, et al. Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2009 Nov 15;49(10):1486-91. 8.Antony JS, Ojurongbe O, van Tong H, et al. Mannose-binding lectin and susceptibility to schistosomiasis. J Infect Dis. 2013. 2013 Jun 1;207(11):1675-83. 9.Maltsev DV, Hurzhii OO. ANA-associated uveitis in the presence of reactivated HHV-7 infection in a patient with MBL deficiency. J Ophthalmol (Ukraine). 2020;6 (497):64–9. 10.Birbian N, Singh J, Jindal SK, et al. Association of the wild-type A/A genotype of MBL2 codon 54 with asthma in a North Indian population. Dis Markers. 2012;32(5):301-8.\ 11.Eurich D, Boas-Knoop S, Morawietz L, et al. Association of mannose-binding lectin-2 gene polymorphism with the development of hepatitis C-induced hepatocellular carcinoma. Liver Int. 2011 Aug;31(7):1006-12. 12.Vengen IT, Madsen HO, Garred P, et al. Mannose-binding lectin deficiency is associated with myocardial infarction: the HUNT2 study in Norway. PLoS One. 2012;7(7):e42113. 13.Christiansen OB, Nielsen HS, Lund M, et al. Mannose-binding lectin-2 genotypes and recurrent late pregnancy losses. Hum Reprod. 2009 Feb;24(2):291-9. 14.Alstadhaug KB, Kvarenes HW, Prytz J, Vedeler C. A case of relapsing-remitting facial palsy and ipsilateral brachial plexopathy caused by HSV-1. J Clin Virol. 2016 May;78:62-5. 15.Tang YW, Cleavinger PJ, Li H, et al. Analysis of candidate-host immunogenetic determinants in herpes simplex virus-associated Mollaret's meningitis. Clin Infect Dis. 2000 Jan;30(1):176–8. 16.Friborg JT, Jarrett RF, Koch A, et al. Mannose-binding lectin genotypes and susceptibility to Epstein-Barr virus infection in infancy. Clin Vaccine Immunol. 2010 Sep;17(9):1484-7. 17.Manuel O, Pascual M, Trendelenburg M, Meylan PR. Association between mannose-binding lectin deficiency and cytomegalovirus infection after kidney transplantation. Transplantation. 2007 Feb 15;83(3):359-62. 18.Valdimarsson H. Infusion of plasma-derived mannan-binding lectin (MBL) into MBL-deficient humans. Biochem Soc Trans. 2003 Aug;31(Pt 4):768-9. 19.Garred P, Pressler T, Lanng S, et al. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr Pulmonol. 2002 Mar;33(3):201-7. 20.Frakking FN, Brouwer NM, van de Wetering D, et al. Safety and pharmacokinetics of plasma-derived mannose-binding lectin (MBL) substitution in children with chemotherapy-induced neutropaenia. Eur J Cancer. 2009 Mar;45(4):505-12. 21.Petersen KA, Matthiesen F, Agger T. Phase I safety, tolerability, and pharmacokinetic study of recombinant human mannan-binding lectin. J Clin Immunol. 2006 Sep;26(5):465-75. 22.Maltsev DV. [The effectiveness of cryopreserved human plasma replacement therapy in patients with primary mannose-binding lectin deficiency suffering from chronic active herpes virus infection]. Immunopatologiia, allergologiia i infektologiia. 2020;2:15-25. Russian. 23.Maltsev DV, Hurzhii OO. Toxoplasma chorioretinitis in primary myeloperoxidase MPO deficiency: A case report. J Ophthalmol (Ukraine). 2019;4:75–81. Conflict of Interest: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|