J.ophthalmol.(Ukraine).2021;5:56-63.
|
http://doi.org/10.31288/oftalmolzh202155663 Received: 26 August 2021; Published on-line: 23 October 2021 EEG rhythms and retinal morphometric parameters before and after conventional therapy with adjunctive neuroprotective agent for refractive amblyopia and strabismic amblyopia I. M. Boichuk, Badri Wael SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: iryna.ods@gmail.com TO CITE THIS ARTICLE: Boichuk IM, Wael Badri. EEG rhythms and retinal morphometric parameters before and after conventional therapy with adjunctive neuroprotective agent for refractive amblyopia and strabismic amblyopia. J.ophthalmol.(Ukraine).2021;5:56-63. http://doi.org/10.31288/oftalmolzh202155663
Background: It has been suggested that the results of traditional pleoptic and orthoptic therapy for amblyopia may be improved by the adjunctive use of neuroprotective agents. Citicoline, a cholinergic and neuroprotective agent, is worthy of attention in this connection. The degree of maturation of the cortical structures can be assessed by electroencephalography (EEG), whereas retinal morphometric parameters, by optical coherence tomography (OCT). Consequently, alpha, beta, delta and theta percent times in the EEG record and OCT-based retinal morphometric parameters may be considered as a potential criterion for assessing the efficacy of treatment for amblyopia. Purpose: To assess EEG rhythms and OCT-based retinal morphometric parameters when using the neuroprotective agent citicoline as an adjunct to treatment for refractive and strabismic amblyopia. Material and Methods: Seventy-nine amblyopic children (158 eyes) aged between 4 and 12 years (were involved in the study. They were divided into a main group of 57 patients treated with conventional therapy plus citicoline eye drops and a control group of 22 patients treated with conventional therapy only. OCT was performed using Stratus OCT 3000 (Carl Zeiss Meditec Inc.) to assess optic disc parameters and retinal nerve fiber layer (RNFL) thickness. The international 10–20 system of electrode placement was employed to perform EEG using an EEG machine (Medicor EEY8S) and a 16-channel computer QUATTOR system (Kharkiv, Ukraine). Patients of the main group were treated with citicoline and vitamin B12 eye drops (OMK2®, one drop thrice daily) as an adjunct to the pleoptic and orthoptic treatment and a month on completion of the pleoptic and orthoptic treatment. This treatment was performed twice a year with a 3-4-month interval between courses. Results: The mean improvement in visual acuity in the amblyopic eye was 0.4 ± 0.16 in the main group versus 0.2 ± 0.1 in the control group. In addition, contrast sensitivity score improved more substantially (from 1.5±0.7 to 2.8±0.4) in the former than in the latter group (from 1.5±0.7 to 2.8±0.4 points). After treatment, the OCT-based temporal RNFL thickness in the main group increased significantly from 72.5±14.6 µm to 78.5±22.0 µm. In addition, alpha indices increased to normal values in 73% of children with refractive amblyopia, and in 55% of children with strabismic amblyopia, of the main group, versus 54% and 60%, respectively, for the control group. Moreover, delta and theta indices reduced to 58±9.6% and 7.8±6.7% in 54% and 60%, respectively, of children of the former group, versus 41.7% and 50%, for the latter group. Therefore, when used as an adjunct to the comprehensive therapy for amblyopia, the neuroprotective agent can improve visual functions and rhythm indices related to EEG rhythms, which facilitates the development of the visual system in this pathology. Keywords: amblyopia, OCT of the retina and optic disc, electroencelography, treatment, neuroprotective agent
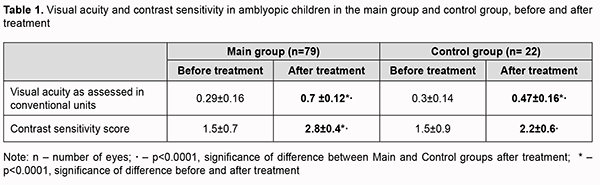
Introduction Amblyopia is a common cause of visual loss in children, accounting for 4-6% of cases of pediatric eye disorders [1, 2]. Findings of recent animal and clinical studies on the pathogenesis of amblyopia allowed concluding that amblyogenic factors prevent a healthy visual pathway from forming between the eye and the brain during the critical period of neurodevelopment [3]. Amblyopia is characterized by histopathological changes in the visual cortex and lateral geniculate nucleus, and the severity of these changes depends on the type of amblyopia. Although mo retinal morphological changes have been found in amblyopic eyes [4, 5], functional changes have been reported in the retinal receptive fields in amblyopic eyes [6-9], and these fields are implicated in contrast sensitivity and form vision. Studies on deprivation found that form vision deprivation affects not so much retinal morphology but rather retinal neurotransmitters, contributing to decreased dopamine and tyrosine hydroxylase synthesis and increased vasoactive intestinal peptide levels. Binocular cortical neurons respond to stimulation of either eye, and Crawford and colleagues [5] reported that the major abnormality in this disease lies in loss of these neurons. It has been experimentally demonstrated that visual cortex plasticity depends on n-methyl-D-aspartate (NMDA) receptors which improve synapse formation. NMDA glutamate receptors mediate visual signal transmission. Acetylcholine, serotonin, and noradrenaline are delivered from the pons to the visual cortex and are involved in visual information transmission to the visual system [10, 11]. Functional abnormalities in amblyopia have been widely studied, and a reduction in practically all visual functions (light sensitivity, color sensitivity, electrophysiological optic nerve function, bioelectrical cortex activity, and etc.) has been demonstrated [12, 13]. Because functional abnormalities of the cortical and subcortical visual system are involved in the etiopathogenesis of amblyopia, EEG studies in amblyopes have been interesting for ophthalmologists for decades. In the 1970s and 1980s, studies by Soviet ophthalmologists [9] investigated EEG rhythm patterns in strabismic patients. In a study by Avetisov and colleagues [1, 2], 82.2% of cases with non-accommodative strabismus showed pathological EEG patterns. They found changes in the alpha rhythm parameters and occurrence of pathologic delta and theta waves in 91 of 110 patients, which indicated damage to the brainstem, basal and pontine structures. They, however, did not mention, whether there were amblyopic children among the study objects. Zislina and Sorokina [15] and Zislina and Shamshinova [16] used visual evoked potential (VEP) response to pattern stimuli and pattern VEP topographic mapping and analyzed the VEP that were extracted from the occipital EEG of patients with refractive and strabismic amblyopia. They demonstrated normal VEP composition and distinct difference in configuration between flash- and pattern reversal evoked-VEP for these patients. Strabismic amblyopia is believed to be the most severe form of amblyopia. Boichuk and colleagues [12, 13] determined the pattern of EEG changes typical of strabismic amblyopia. These abnormalities can be detected either in the hemisphere contralateral to the amblyopic eye or in both brain hemispheres based on the background EEG data and EEG response to functional load like the absence of eye opening response and rhythmic driving response to unilateral and bilateral photic stimulation and occurrence of bilaterally asymmetrical and synchronous theta and delta waves in occipital and frontal responses (theta and delta percent time of 46-48% can be used as a criterion of the amblyopia-related abnormality located in the visual cortex and in the cortical and subcortical visual system). Defects in visual functions in amblyopic eyes may have a neuroretinal explanation. Studies by Lempert [17, 18] used optic disc rim areas, corrected for magnification, retinal areas, and a derived ratio, retinal area/disc rim area (RetA/DRimA) as main outcome measures, and found, that the RetA/DRimA of the fellow eyes was smaller than for the amblyopic but larger than that of the normal eyes. These differences were due to smaller optic disc rim areas in the amblyopic and fellow eyes, leading to expansion of the retinal receptive field. It was concluded that amblyopic and their fellow eyes, when compared with normal eyes, have reduced innervations of comparable retinal areas. These differences can be attributed to a paucity of nerve fibers, as indicated by the smaller neuroretinal rim areas. Therefore, optical coherence tomography (OCT) data can be used in diagnostic assessment as well as the assessment of retinal changes in various forms of strabismic amblyopia. It has been reported that the use of some medications as adjuncts to conventional treatment can increase brain sensitivity to treatment and improve treatment response in amblyopia [10, 19-22]. Citicoline is a complex biomolecule that plays a role in cellular metabolism [10, 20] and might be used to enhance the transmission of pulses in the retina. It is a mononucleotide, a choline precursor and serves as a choline donor in acetylcholine biosynthesis. Citicoline is involved in the renewal and synthesis of the phospholipids of cell membranes and neuromediators such as acetylcholine and dopamine. Phospholipid synthesis and renewal provide a basis for neuroprotection [11, 19, 23]. Citicoline decreases phospholipase stimulation, thus preventing apoptotic and necrotic cell death [11, 23], and serves as a factor of growth of optic nerve fibers in degenerative eye disease [20]. Experimental findings [24, 25] implicate that dendritic growth may be impaired due to inadequate light stimulation of the retina during the development of inner and outer retinal layers. In such cases, dendrites of adjacent cells may fill the gap, leading to displacement of relevant retinal cells within the nasal and temporal retina or vertical retina. Therefore, analysis of the changes between pre and post-treatment OCT-based morphometric parameters of retinal and optic disc structures should allow the objective assessment of the efficacy of the proposed treatment of amblyopia. Alpha, beta, delta and theta percent times, frequencies and amplitudes, and the level of alpha-rhythm suppression are essential for characterizing the function of the central visual system [26-29]. Conventional EEG is recorded in within a limited band of frequencies (0.3-50 Hz). Brain rhythms recorded in EEG fall into the following ranges: delta (0.3-4 Hz), theta (4-8 Hz), alpha (8–13 Hz), beta 1 or low frequency beta (13-25 Hz), beta 2 or high frequency beta (25-35 Hz), and gamma or beta 3 (35-50 Hz) [29, 30]. Brain’s delta activity, theta activity, alpha activity, beta activity and gamma activity correspond to these rhythms. The lower the frequency and the higher the amplitude, the more severe is the pathological process. Alpha-rhythm reflects the degree of maturation of the cortical structures [31], whereas the occurrence of rhythmic slow activity (theta and delta percent time > 10%) indicates immature brain structures [32, 38]. Therefore, EEG profiles before and after treatment may be used as a criterion for assessing therapy outcomes [30]. The purpose of this study was to assess EEG rhythms and OCT-based retinal morphometric parameters when using the neuroprotective agent citicoline as an adjunct to comprehensive treatment for refractive and strabismic amblyopia. Material and Methods Seventy-nine amblyopic children (158 eyes) aged between 4 and 12 years (mean age, 7.2 ± 2.1 years) were involved in the study. They were divided into a main group of 57 patients (37 children with refractive amblyopia and 20 with strabismic amblyopia) treated with conventional therapy plus citicoline eye drops and a control group of 22 patients (12 children with refractive amblyopia and 10 with strabismic amblyopia) treated with conventional therapy only. Ocular examination included best-corrected visual acuity (Sivtsev chart), refractometry, and contrast sensitivity score for near as assessed using the advanced Bausch & Lomb charts (2013) [33], and was conducted before and after treatment. OCT was performed using Stratus OCT 3000 (Carl Zeiss Meditec Inc.) to assess morphometric parameters of the retina and optic disc. Scans were obtained using standard protocols. Optic nerve characteristics (total optic disc area, cup area, neuroretinal rim area, and cup/disc ratio), retinal nerve fiber layer (RNFL) thickness in four quadrants, and mean RNFL thickness for the amblyopic eye and fellow eye were determined. The international 10–20 system of electrode placement was employed to perform EEG using an 8-channel EEG machine (Medicor EEY8S) and a 16-channel computer QUATTOR system (Kharkiv, Ukraine). Alpha, beta, delta and theta percent times were determined. Children of the control group received conventional pleoptic and orthoptic treatment courses only. Patients of the main group were treated with citicoline and vitamin B12 eye drops (OMK2®, one drop thrice daily) as an adjunct to the pleoptic and orthoptic treatment and a month on completion of the pleoptic and orthoptic treatment. This treatment was performed twice a year with a 3-4-month interval between courses. No side effect was observed in any case. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Statistica 8.0 (StatSoft, Tulsa, OK, USA) software was used for statistical analysis. Mean (M) and standard deviations (SD) were calculated for quantitative variables. The level of significance p ≤ 0.05 was assumed. Multiple post hoc comparisons were performed using the Newman–Keuls correction or chi2 test for contingency table analysis. Results In the total study sample, the mean baseline uncorrected visual acuity (UCVA) and best-corrected visual acuity (BCVA) in the amblyopic eye were 0.22 ± 0.2 and 0.3 ± 0.2, respectively, and the mean BCVA in the fellow eye was 0.8 ± 0.2. Based on the static refraction measurements under cycloplegia in the amblyopic eye, mild hyperopia was found in 64.7%, moderate hyperopia, in 25.4%, and severe hyperopia, in 5.9% of study patients. Mild or moderate hyperopia (mean refractive error, 2.25 ± 1.8 D) was found in the fellow eyes. Amblyopes had central fixation in the ambyopic eye in monocular viewing. The deviation angle in strabismic amblyopic eyes ranged from 8 to 12 degrees. After treatment, an improvement in visual functions was found in both groups (р < 0.05). Particularly, the mean improvement in BCVA in the amblyopic eye was 0.4 ± 0.16 in the main group (with the neuroprotective agent used as an adjunct to traditional treatment) versus 0.2 ± 0.1 in the control group (traditional treatment only). In addition, contrast sensitivity score improved more substantially (from 1.5±0.7 to 2.8±0.4) in the former than in the latter group (Table 1).
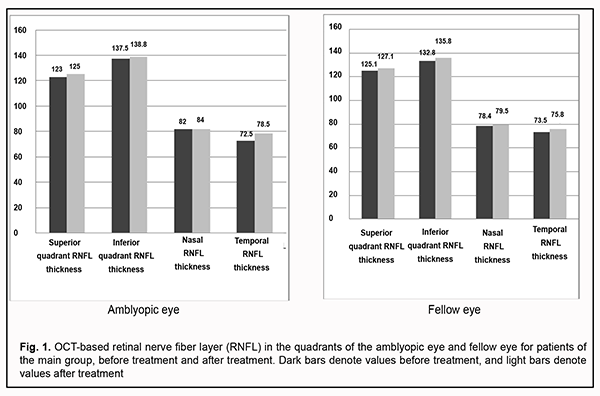
OCT-based RNFL thickness in four quadrants for the amblyopic eye and fellow eye before and after treatment are presented in Fig. 1.
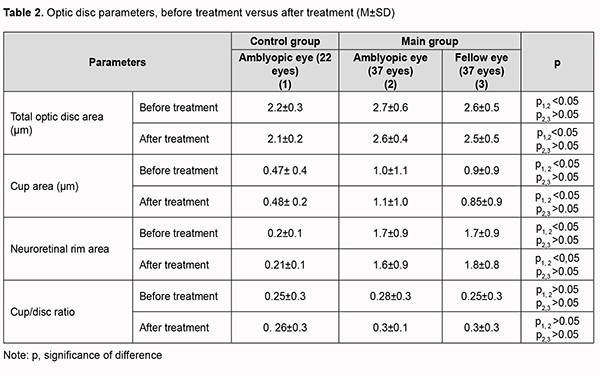
Mean baseline RNFL in patients of the main group was thicker than the age norm primarily due to RNFL thickness in the inferior quadrant (137.5±27.3 µm for the amblyopic eye and 132.8±23.2 µm for the fellow eye) and nasal quadrant (82.0 ± 22.8 µm for the amblyopic eye and 78.4 ± 19.4 µm for the fellow eye). The temporal quadrant had the thinnest RNFL thickness among all four quadrants. After treatment, the RNFL thickness in the inferior and nasal quadrants decreased, and in the superior and nasal quadrants increased, but not statistically significantly, whereas in the temporal quadrant increased significantly from 72.5±14.6 µm to 78.5±22.0 µm, which may indicate an increase in the interneuronal connections in the retina. No significant difference between post-treatment and baseline data for other quadrants was observed. To the best of our knowledge, there have been no studies on post-treatment changes in OCT-based RNFL thickness in amblyopic and fellow eyes. There was no significant change in OCT-based morphometric parameters of the retina in eyes of the control group after treatment. Table 2 shows OCT-based parameters of the optic nerve in eyes of both groups after treatment.
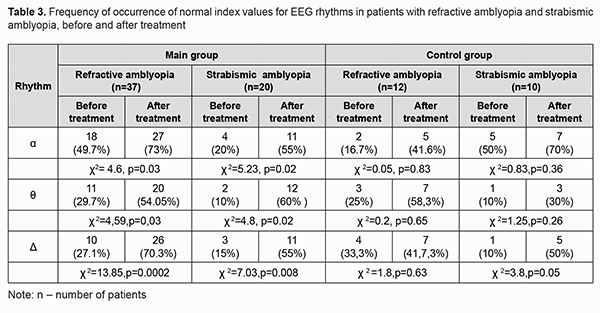
The baseline optic disc area was significantly larger, and the mean baseline RNFL thickness was thicker (primarily due to the inferior and nasal quadrants) in the amblyopic eye in the main group than in the control group (p < 0.05). After treatment, no significant change in OCT-based parameters of the optic nerve or RNFL thickness in the amblyopic eyes and fellow eyes of either group was observed. We also assessed EEG profiles of patients before and after treatment of amblyopia. The alpha rhythm is one of the most prominent normal brain rhythms, mostly defined by its frequency of 8 to 13 Hz, and has a peak-to-peak amplitude of 30 to 80 μV (most commonly, 40 to 60 μV) for bipolar central-occipital EEG. Table 3 presents pre-treatment and post-treatment numbers and percentages of patients with normal EEG alpha, delta and theta indices for the main group and controls.
High frequency beta index was within normal range (3.5 ± 2.5%) and did not change significantly in either group after treatment. In most children with amblyopia (53%), baseline alpha index was below the normal range, with a mean value of 14.6±12.3%. In addition, mean baseline delta and theta indices were 70.1 ± 9.9% and 11.1 ± 8.7%, respectively, which was above the normal range. This may indicate immature cortical electrogenesis as well as the patient’s inadvertence during EEG recording [13, 14, 32, 34]. Baseline delta indices were lower, whereas baseline theta indices higher in children with refractive amplyopia compared to those with strabismic amblyopia (55.2% vs 74.8% and 13.6% vs 7.1%, respectively; р < 0.05). After treatment with adjunct neuroprotective agent, alpha index became normal in 73% of children with refractive amblyopia, and in 55% of children with strabismic amblyopia, and was 15.2 ± 8.6% (at an amplitude of 65 ± 10.2 µV). We have previously reported that alpha index ranged from 15% to 30% in children with amblyopia [13]. In the current study, in most children with amblyopia (53%), baseline alpha index was below the normal range, with a mean value of 14.6±12.3%. In addition, mean baseline delta and theta indices were 70.1 ± 9.9% and 11.1 ± 8.7%, respectively, which was above the normal range (22.5±10.3%) [32, 34]. After comprehensive treatment with adjunct neuroprotective agent, alpha index became normal (14.6 ± 12.3%) in 27 of 37 of children (73%). In addition, delta and theta indices decreased to 58±9.6% and 7.8±6.7%, respectively, indicating improved function of the visual system and a tendency towards normal electrogenesis of cortical neurons, which was accompanied clinically by an improvement in visual acuity. After treatment, alpha index became normal in 9 of 22 children with refractive amblyopia (41.6%) of the control group (versus 73% for the main group, χ2=6.0, р=0.01). In addition, delta and theta indices decreased and became normal in 54% and 60% of children, respectively, of the main group. Moreover, delta and theta indices decreased and became normal in 58% and 30%, and 33% and 50%, of children with refractive amblyopia and strabismic myopia, respectively, of the control group. Discussion It can be concluded from the literature that RNFL thickness measurements do not tend to be affected by eccentric fixation [24, 25, 35-40], and this morphometric index is reproducible and objective. It has been demonstrated that refractive error of the eye is associated with RNFL, and that RNFL is thicker in hyperopes than in emmetropes and thinner in myopes than in emmetropes [18, 38, 41, 42]. Although many others [34, 36, 37] have noted RNFL thickness vary between eyes with different types of amblyopia [38, 41, 42], the effect of a neuroprotective agent on changes in retinal thickness has not been studied until now. The current study found that the neuroprotective agent as an adjunct in the comprehensive treatment of amblyopia increased temporal RNFL thickness from 72.5±14.6 µm at baseline to 78.5±22.0 µm, which may indicate an increase in the interneuronal connections in the retina. Alpha-rhythm reflects the degree of maturation of the cortical structures [31], whereas the occurrence of rhythmic slow activity with theta and delta percent time > 10% indicates immature brain structures [32, 38]. EEG studies [16, 38] have demonstrated that there is a gradual transition from low-frequency brain rhythms to those of a higher frequency in phylogenesis [32]. To the best of our knowledge, this is the first study to investigate alpha, beta, delta and theta percent times before and after application of a neuroprotective agent as an adjunct in the treatment of various types of amblyopia. We found the neuroprotective agent to be a beneficial adjunct in the comprehensive treatment of children with amblyopia, with (a) increased to normal alpha index values in 73% of children with refractive amblyopia, and in 55% of children with strabismic amblyopia, versus 41.6% for children treated with conventional therapy only, and (b) decreased delta and theta index values (to 58±9.6% and 7.8±6.7%, respectively) in 54% of children with refractive amblyopia, and in 60% of children with strabismic amblyopia, versus 41.7% and 50%, respectively, for children treated with conventional therapy only. In addition, improvements in visual acuity and contrast sensitivity were more marked in amblyopic patients treated with adjunctive neuroprotective agent than in those treated with conventional therapy only. Therefore, the neuroprotective agent as an adjunct in the comprehensive treatment of amblyopia increased temporal RNFL thickness and improved interneuronal connections in the retina and rhythm indices related to EEG rhythms, which facilitated the development of the visual system and was accompanied by an improvement in visual acuity. References 1. Avetisov ES. [Strabismic amblyopia and its management]. Moscow: Meditsina; 1968. Russian. 2.Avetisov SE. Kashchenko TP, Shamshinova AM., editors. [Visual functions and their correction in children]. Moscow: Meditsina; 2006. Russian. 3. Demer JL, von Noorden GK, Volkow ND, Gould KL. Imaging of cerebral blood flow and metabolism in amblyopia by positron emission tomography. Am J Ophthalmol. 1988 Apr 15;105(4):337-47. 4. Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377-409. 5. Crawford MLJ, von Noorden GK, Meharg LS, et al. Binocular neurons and binocular function in monkeys and children. Invest Ophthalmol Vis Sci. 1983 Apr;24(4):491-5. 6. Ibatulin RA. [Visual functions in amblyopia as assessed by psychophysical and electrophysiological studies]. Thesis of dissertation for the degree of Dr Sc (Med). Moscow: Helmholtz Research Institute of Eye Diseases; 1998. Russian. 7.Ikeda H. Visual acuity, its development and amblyopia. J R Soc Med. 1980 Aug;73(8):546-55. 8. Ikeda H, Wright M.J. Properties of LGN cells in kittens reared with convergent squint: a neurophysiological demonstration of amblyopia. Exp Brain Res. 1976;25(1):63-77. 9. Campos EC. Future directions in the treatment of amblyopia. Lancet. 1997 Apr 26;349(9060):1190. 10. Campos EC, Schiavi C, Benedetti P, et al. Effect of citicoline on visual acuity in amblyopia: preliminary results. Graefes Arch Clin Exp Ophthalmol. 1995 May;233(5):307-12. 11. Frolov MA, Morozova MS, Frolov AM, et al. [Citicoline: prospects for the use in primary open angle glaucoma]. Rossiiskii oftalmologicheskii zhurnal. 2011;4:108-12. Russian. 12. Boichuk IM. [Value of EEG in determining binocular interaction in children with refractive and strabismic amblyopia]. Oftalmol Zh. 2001;4:18-22. Russian. 13. Boichuk IM. [Visual functions in children with refractive and anisometropic amblyopia]. Odesskii meditsinskii zhurnal. 2003;6:50-4. Ukrainian. 14. Dobromyslov AN. [On the conditional reflex-associated nature of binocular vision and concomitant strabismus]. Oftalmol Zh. 1963;3:160-5. Russian. 15. Zislina NN, Sorokina RS. [The effect of functional and organic disorders in the visual system on the amplitude-temporal characteristics of evoked potentials]. Fiziol Cheloveka. 1991 May-Jun;17(3):27-33. 16. Zislina NN, Shamshinova AM. [Physiological basics and potential for application of visual evoked potentials in differential diagnosis of eye disease]. In: [Clinical physiology of vision]. Moscow: Rusomed; 1993. p. 146-57. Russian. 17. Lempert P. Optic nerve hypoplasia and small eyes in presumed amblyopia. J AAPOS. 2000 Oct;4(5):258-66. 18. Lempert P. Retinal Area and Optic disk rim area in Amblyopic, Fellow, and Normal Hyperopic Eyes: A Hypothesis for Decreased Acuity in Amblyopia. Ophthalmology. 2008 Dec;115(12):2259-61. 19. Stavitskaia TV. [Experimental clinical study of pharmacokinetic and pharmacodynamic aspects of neuroprotective therapy in ophthalmology]. Thesis of dissertation for the degree of Dr Sc (Med). St Petersburg: Kirov Military Medical Academy; 2005. Russian. 20. Khanlarova NA, Gadjiyeva NR, Guliyeva VV, Guliyeva TD. [Efficacy of an ophthalmic neuroprotector as an adjunct to the comprehensive treatment of amblyopia in children]. Oftalmologiia. 2015;3(19):87-91. Russian. 21. Gandolfi S, Marchini G, Caporossi A, Scuderi G, Tomasso L, Brunoro A. Cytidine 5’-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients. 2020 Mar 18;12(3):793. 22. Carnevale C, Manni G, Roberti G, Micera A, et al. Human vitreous concentrations of citicoline following topical application of citicoline 2% ophthalmic solution. PLoS ONE. 2019 Nov 14;14(11):e0224982. 23. Morozova NS. [Effect of neuroprotective therapy on factors of apoptosis in glaucomatous optic neuropathy]. Thesis of dissertation for the degree of Dr Sc (Med). Moscow: Helmholtz Research Institute of Eye Diseases; 2013. Russian. 24. Kee SY, Lee SY, Lee YC. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006 Sep;20(3):177-81. 25. Miki A, Shirakashi M, Yaoeda K, Kabasava Y. Retinal nerve fiber layer thickness in recovered and persistent amblyopia. Clin Ophthalmol. 2010;4: 1061-4. 26. Zenkov LR. [Electroencephalography (with Elements of Epileptology)]. Taganrog: TGRU; 1996. p.22-99. Russian. 27. Zinchenko VP, Vdovina LI, Gordon VM. [Study on the functional structure of combinatory problem solving]. In: [Motor components of vision]. Moscow: Nauka; 1975. Russian. 28. Kustubaieva AM. [Age-related dynamics of brain’s rhythms of electrical activity. Anxiety level and EEG indices]. Eksperimentalnaia psychologiia. 2012;5(3):5-20. Russian. 29. Novikov SI. [EEG rhythms and cognitive processes]. Sovremennaia zarubezhnaia psychologiia. 2015;4(1):91-108. Russian. 30. Omelchenko VP, Mikhalchich IO. [Non-linear analysis of human EEG rhythmic components in health]. Izvestiia IuFU. Tekhnicheskiie nauki. 2014;159(10):52-10. Russian. 31. Gomez MN. [Morphological bases of the evolution of the E.E.G. in man. I. Relation between weight of the brain and the E.E.G. frequency from the 1st 6 postnatal months until 9 years of age]. Arch Neurobiol (Madr). May-Jun 1976;39(03):195-212. 32. Galkina NS. [Electroencephalograms of children in health and disease. Clinical electroencephalography]. Moscow: Meditsina; 1973. p.270-285. Russian. 33. Contrast Sensitivity. Contrast Measurement Scales (Weber Contrast). Available at: www/precision – vision.com/index.cfm/feature/12. 34. Botabekova TK, Kurgambekova NS. [Optical coherent tomography in the diagnosis of amblyopia]. Vestn Oftalmol. Sep-Oct 2005;121(5):28-9. 35. Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006 Aug;142(2):247-51. 36. Savini G, Zanili M, Carelli V. Correlation between retinal nerve fibre layer thickness and optic nerve head size: an optical coherence tomography study. Br J Ophthalmol. 2005 Apr;89(4):489-92. 37. Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004 Jul;45(7):2224-30. 38. Yoon SW, Park WH, Baek SN, Kong SM. Thickness of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005 Mar;19(1):62-7. 39. Weinreb RN. Glaucoma neuroprotection. What is it? Why is it needed? Can J Ophthalmol. 2007 Jun;42(3):396-8. 40. Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56(9):637-60. 41. Boichuk IM, Ivanytska EV. [Results of optical coherence tomography of the retina and optic nerve in children with unilateral amblyopia]. In: [Proceedings of the 3rd conference on the current issues of medical and social rehabilitation of children with a disabling eye condition]. Evpatoriia, 4-6 October, 2006. Russian.
42. Boichuk IM, Ivanytska EV. [Results of optical coherence tomography of the retina and optic nerve in children with high unilateral amblyopia]. Oftalmol Zh. 2006;3:46-9. Russian.
Conflict of Interest: Authors declare that there are no conflicts of interest that might influence their opinion on the subject matter or materials described or discussed in this manuscript.
|