J.ophthalmol.(Ukraine).2021;5:41-46.
|
http://doi.org/10.31288/oftalmolzh202154146 Received: 24 March 2021; Published on-line: 23 October 2021 Clinical features of the course of optic neuritis as a complication of idiopathic anterior uveitis L. V. Venger 1, V. V. Savko 2, O. V. Kovtun1, V. M. Sokolov 1 1 Odesa National Medical University;Odesa (Ukraine) 2 SI «The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine»; Odesa (Ukraine) TO CITE THIS ARTICLE: Venger LV, Savko VV, Kovtun OV, Sokolov VM. Clinical features of the course of optic neuritis as a complication of idiopathic anterior uveitis. J.ophthalmol.(Ukraine).2021;5:41-6. http://doi.org/10.31288/oftalmolzh202154146 Background: Uveitis is the fifth leading cause of visual impairment in developed countries and responsible for about 20% of legal blindness. A serious complication of anterior uveitis, optic neuritis, as well as the factors contributing to its development has not been investigated completely, but its early diagnosis is a challenge. Purpose: To reveal the features of the clinical course of optic neuritis as a complication of idiopathic anterior uveitis. Material and Methods: This study involved 150 patients with unilateral idiopathic anterior uveitis who were examined and treated at the Filatov Institute. Of these, 114 did not have signs of optic neuritis, and 34 had optic neuritis in the presence of uveitis. Patients underwent an eye examination including visual acuity, ophthalmoscopy, biomicroscopy, intraocular pressure (IOP) measurement, and Humphrey perimetry. Treatment involved antibiotics, non-steroidal anti-inflammatory drugs, immune suppressors, corticosteroids, and biological immune response modulators. Results: The clinical features related to inflammation were more severe in the group of patients with optic neuritis as a complication of anterior uveitis than in the group of patients with uncomplicated uveitis. Particularly, the number of keratic precipitates was high in 69.7%, hypopyon was present in 83.3%, and vitreous haze was intensive or apparent in all patients of the former group, versus no patients, one patient and 4.4% of patients, respectively, of the latter group. The number of keratic precipitates was moderately positively correlated with the development of optic neuritis in patients with anterior uveitis, (Spearman ρ, 0.566; p < 0.05). Concomitant otorhinolaryngological and odontogenic inflammatory diseases were found in 82.5% of patients with anterior uveitis. There was a significant positive association between the presence of concomitant otorhinolaryngological and odontogenic inflammatory diseases and the development of optic neuritis in patients with anterior uveitis (χ2=5.50, p=0.0191). Keywords: idiopathic anterior uveitis, complications, optic neuritis, clinical features
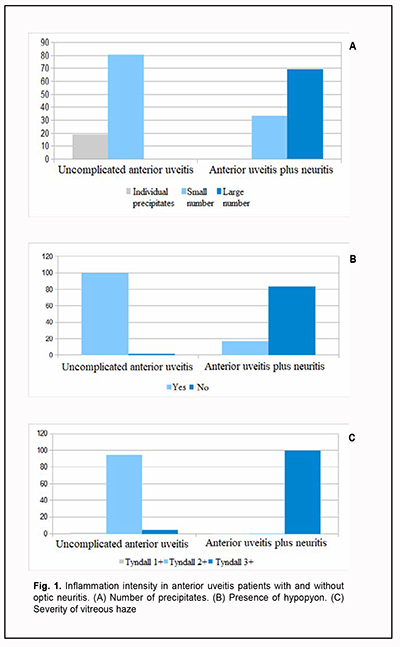
Introduction Uveitis is the fifth leading cause of visual impairment in developed countries and responsible for about 20% of legal blindness [1], with anterior uveitis being the most common form of uveal inflammation. Studies vary with regard to the reported frequencies of different forms of uveitis on the basis of anatomical location, nature and duration of the disease [2, 3]. Particularly, a study by Arbenyeva and colleagues [4] included 226 patients with uveitis, with the percentages of patients with anterior uveitis, intermediate uveitis, posterior uveitis and panuveitis being 61%, 2%, 34%, and 3%, respectively. Шт In addition, in a study by McCannel et al. [5], anterior uveitis was the most common and accounted for 60.6% of all cases, followed by posterior uveitis (14.6%), intermediate uveitis (12.2%), and panuveitis (9.4%). In both the above studies, anterior uveitis was significantly more common than other forms of the disease. Chronic uveitis is more common than acute uveitis. Nongranulomatous disease is more common than granulomatous disease, and non-infectious disease is more common than infectious disease among patients with anterior uveitis. In addition, a systemic disease has been found in as much as 30% of patients with anterior uveitis [6]. Establishing a definitive etiology of uveal tract inflammation is not always possible. Uveitic is idiopathic in up to 40% of cases [7]. Intraocular inflammation is frequently caused by inflammation of the meninges of the brain and/or spinal cord (arachnoiditis), paranasal sinus disease, caries, tooth root granuloma, or periodontosis. Uveal tract inflammation most commonly occurs when there is compromised ocular coat integrity, post-traumatic or post-surgical breakdown of the blood-ocular barrier, in contact sinusitis, sphenoiditis or odontogenic infection, in the presence of exogenous and endogenous factors of infectious or non-infectious origin. Macular edema is a complication found in 30% of patients with idiopathic anterior uveitis, frequently leading to irreversible loss of vision [8, 9]. The diagnosis of optic neuritis as a complication of anterior uveitis is a challenge because the fundal view is limited by the presence of exudates in the anterior chamber and vitreous as well as pupillary block. Although as early as 1968 Duke-Elder supposed that ophthalmologists much more often fail than manage to find the involvement of the retina and optic nerve in inflammation, diagnosing optic neuritis is still difficult, which may explain a wide variation in the reported rate of this complication of idiopathic anterior uveitis, ranging from 5% to 65% [10, 11]. In addition, the factors contributing to the development of optic neuritis in patients with anterior uveitis have not been investigated completely. The purpose of this study was to reveal the features of the clinical course of optic neuritis as a complication of idiopathic anterior uveitis. Material and Methods This open-label, non-interventional study was conducted within the framework of Optimizing the Diagnosis, Treatment and Prevention of the Development of Ocular Degenerative and Inflammatory Disorders, a research program (Ukrainian State Registration No. 0119 U 003575). The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Informed consent was obtained from all patients involved in the study. This study involved 150 patients (94 men and 56 women; age, 18 to 83 years) with unilateral idiopathic anterior uveitis who were examined and treated in the Department of Ocular Inflammation, the Filatov Institute of Eye Disease and Tissue Therapy, and Medical Eye Care Center of Odesa National Medical University. The inclusion criterion was the presence of unilateral idiopathic anterior uveitis. The exclusion criteria were diabetes; acute infectious, viral, or cardiovascular disease; abnormal circulation in the major ocular vessels; history of ocular surgery; or pregnancy. Anterior uveitis was diagnosed using International Classification of Diseases, 10th Revision (ICD-10) criteria and based on the Standardization of Uveitis Nomenclature criteria, categorizing uveitis along several dimensions: course, laterality, anatomic location of the inflammation, morphology, and presence of active infection [12]. Patients underwent a routine eye examination (ophthalmoscopy, biomicroscopy, intraocular pressure (IOP) measurement, and Humphrey perimetry). Best-corrected visual acuity (BCVA) was assessed at 6 m using charts composed of Ukrainian letter optotypes. Standardization of the 2005 Uveitis Nomenclature (SUN) working group classification was used to classify the uveitis and grade the uveitis activity [13, 14]: Grade 0 (no changes); Tyndall 1+ (faint changes, tiny free-floating opacities (0-10), fundus examination shows no haze); Tyndall 2+ (free-floating vitreous opacities with collagen fibril separation (2-20 cells in the field), very flare haze (21-50 cells in the field), iris and lens details clear and optic disc and vessels clear); Tyndall 3+ (intense opacities, iris and lens details hazy, and optic disc and vessels hazy); Tyndall 4+ (intense inflammation, hypopyon in the anterior chamber, ophthalmoscopy is difficult to perform, or fundus details are not clear). The patients were divided into two groups: those with anterior uveitis only and those with anterior uveitis complicated by optic neuritis. Neuritis was diagnosed in 36 patients. Particularly, 2 patients had peripheral neuritis, 3 patients, axial neuritis, and 31, transverse neuritis. In 114 patients with uveitis only, anterior uveitis pursued an acute course with sudden onset and limited duration (< 3 months). Among 36 patients with neuritis, 23 had a chronic course (12 months), and the rest had no recurrence within a year. Visual acuity in the healthy fellow eye ranged from 0.6 to 1.0. In 114 patients with uveitis only, visual acuity in the affected eye ranged from 0.3 to 0.5 in twenty-five and from 0.5 to 0.9 in the rest. Among 36 patients with neuritis, visual acuity in the affected eye was high in one, ranged from 0.3 to 0.5 in 22, and ranged from 0.12 to 0.25 in 13. The IOP was normal in all patients. Patients received treatment as per the protocol adopted by the Ethics Commission of the Filatov institute (2012) and approved by the National Academy of Medical Science of Ukraine (2018) on the basis of United States Public Health Service (USPHS)/ Infectious Diseases Society of America (IDSA) Guidelines for the Prevention of Opportunistic Infections in Persons Infected with Human Immunodeficiency Virus. Treatment involved antibiotics, non-steroidal anti-inflammatory drugs, immune suppressors, corticosteroids, and biological immune response modulators. Treatment duration was 10 days or less for patients with uncomplicated anterior uveitis, and 13.5 days or less for patients with anterior uveitis complicated by optic neuritis. Optic nerve atrophy was diagnosed in 30 of 36 patients with neuritis. Particularly, 16 patients (53.3%) exhibited optic nerve atrophy at six months or earlier and 14 patients (46.7%), at 12 months. Statistical analyses were conducted using Statistica 10.0 (StatSoft, Tulsa, OK, USA) software. Pearson chi-square test and Spearman rank correlation coefficient were used to assess associations between study parameters [15]. Results We have previously reported on visual acuity in patients with anterior uveitis and found that visual functions were reduced in those with uveitis complicated by optic neuritis. This reduction in visual acuity was substantial, by 0.12 to 0.25, in 36.1% of patients. There was a moderate positive Spearman correlation (r = 0.554, p <0.05, n = 150) between optic neuritis in the presence of anterior uveitis and reduced visual acuity. The following is noteworthy with regard to clinical features of patients with optic neuritis developing in the presence of anterior uveitis. Generally, anterior uveitic eyes were characterized by the presence of mixed conjunctival injection, exudate in the anterior chamber, posterior synechiae, corneal endothelial precipitates, vitreous haze, and hypopyon. We compared anterior uveitis patients with versus without optic neuritis with regard to the clinical picture. Inflammation severity was assessed using scores for standard clinical signs such as the number of keratic precipitates, presence of hypopyon, and severity of vitreous haze, which were significantly positively correlated with each other, with a Spearmen's correlation coefficient ranging from 0.574 to 0.789 (p < 0.05, n = 150). The inflammatory reaction was more marked in patients with optic neuritis as a complication of anterior uveitis. Thus, the number of keratic precipitates was high in 24 (69.7%), moderate in 12 (33.3%), and low in zero patients of this group, versus zero, 93 (80.7%) and 22 (19.3%) patients, respectively for the group of anterior uveitis patients without neuritis (Fig. 1A).
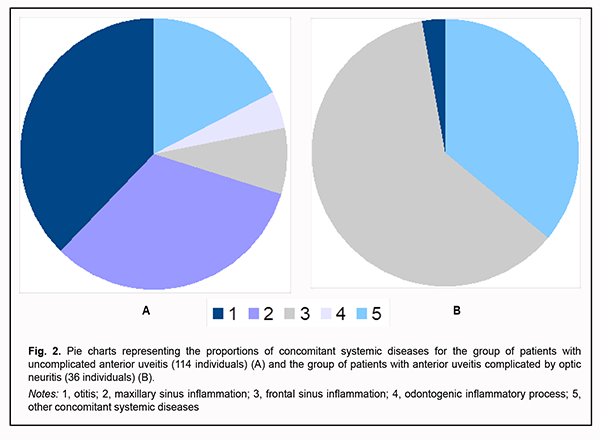
Hypopyon was found in one anterior uveitis patient (0.9%) without optic neuritis versus 30 anterior uveitis patients (83.3%) with optic neuritis (Fig. 1 B). Each patient with anterior uveitis had some degree of vitreous haze ranging from collagen fiber separation to fibrous cords. In addition, each patient with anterior uveitis complicated by optic neuritis had marked or intense flare (Tyndall 3+ to Tyndall 4+). Of the patients with anterior uveitis without optic neuritis, one (0.9%) had faint flare (Tyndall 1+), five (4.4%), marked or intense flare, but most (108; 94.7%) had moderate flare (Tyndall 2+) (Fig. 1 C). The number of keratic precipitates was the only sign of inflammation severity that correlated with the development of optic neuritis in patients with anterior uveitis, and this was a moderate positive correlation (Spearman correlation coefficient, 0.566; p < 0.05, n = 150). Histories of study patients were significant for systemic diseases. In patients with uncomplicated uveitis, the most common systemic comorbidity was otitis (43 patients; 37.7%), followed by maxillary sinus inflammation (37 patients, 32.5%), frontal sinus inflammation (9 patients, 7.9%), odontogenic inflammatory process (5 patients, 4.4%), and other systemic diseases (20 patients; 17.5%) (Fig. 2A). Of the patients with anterior uveitis complicated by optic neuritis, one (2.8%) had otitis, 22 (61.1%), frontal sinus inflammation, and the rest 13 patients (36.1%), other systemic diseases. Therefore, most anterior uveitis patients with and without optic neuritis (63.9% and 82.5%, respectively) had otorhinolaryngological inflammatory diseases (Fig. 2 B).
Discussion Pearson’s chi-squared analysis found that there was a significant association of the concomitant head and neck inflammatory disease (otitis, maxillary sinus inflammation, frontal sinus inflammation, or odontogenic inflammatory process) and the development of optic neuritis in patients with anterior uveitis (χ2=5.50, p=0.0191). We confine our interest to studies that aim to identify the pathogenetic and clinical features of optic neuritis in the presence of anterior uveitis in an attempt to find effective methods of early diagnosis of this serious complication. This is important, because uveitis accounts for 5% to 15% of all cases of eye disease, affecting most commonly young adults (with a mean age at onset of 30.7 years) [16]. In addition, optic neuritis as a complication of anterior uveitis is a significant cause of visual impairment and blindness [17]. Optic neuritis is a major optic nerve disease, accounting for 30% to 40% of all cases [18, 19]. Our findings on the clinical course anterior uveitis are in agreement with the findings of other clinical studies. The presence of pericorneal injection, keratic precipitates, exudative response in the anterior chamber aqueous (various grades of anterior chamber flare), the presence of hypopyon or fibronectin in apparent inflammation, and vitreous haze are considered typical features [20-22]. We found visual acuity in patients with optic neuritis in the presence of anterior uveitis to be especially reduced, which is also in agreement with the findings of others. In the Optic Neuritis Treatment Trial [23], 95% of patients showed unilateral vision loss and 92% had associated retroorbital pain that frequently worsened with eye movement. The importance of our finding of an association of anterior uveitis and its complication, optic neuritis, with the presence of concomitant otorhinolaryngological and odontogenic inflammatory diseases is confirmed by the conclusion of others on the importance of identification of major systemic diseases for the diagnosis and treatment of optic neuritis [24]. Therefore, understanding the features of the course of uveal tract inflammation and complications occurring in patients with anterior uveitis is a prerequisite for attempting to find effective methods of early diagnosis of optic neuritis in anterior uveitis patients and opportunity for early pathogenetically oriented therapy with a potential for restoration of vision and avoidance of recurrent inflammation. Conclusion First, it was demonstrated that the clinical features related to inflammation were much more severe in the group of patients with optic neuritis as a complication of anterior uveitis than in the group of patients with uncomplicated uveitis. Particularly, the number of keratic precipitates was high in 69.7%, hypopyon was present in 83.3%, and vitreous haze was intensive or apparent in all patients of the former group, versus no patients, one patient and 4.4% of patients, respectively, of the latter group. Second, the number of keratic precipitates was moderately positively correlated with the development of optic neuritis in patients with anterior uveitis (Spearman correlation coefficient, 0.566; p < 0.05, n = 150). Finally, concomitant otorhinolaryngological and odontogenic inflammatory diseases were found in 82.5% of patients with anterior uveitis. There was a significant association between the presence of concomitant otorhinolaryngological and odontogenic inflammatory diseases and the development of optic neuritis in patients with anterior uveitis (χ2=5.50, p=0.0191).
References 1. Fardeau C, Champion E, Massamba N, Lehoang P. Uveitic macular edema. Eye. 2016; 30(10):1277–92. 2. Anesi SD, Foster CS. Anterior uveitis: etiology and treatment. Advanced Ocular Care. 2011;2(1):32–4. 3. Miserocchi E, Fogliato G, Modorati M, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013; 23: 705- 717. 4. Arbenyeva NS, Chekhova TA, Bratko GV, Chernykh VV. [Comparative analysis of the incidence of patients with uveitis]. In: [Current issues of ophthalmology: a collection of science works. Proceedings of the 7th National Russian Conference of Young Scientists]. Editor, B.E. Malyugin. Moscow: Ophthalmology; 2012. p. 28–9. Russian. 5. McCannel CA, Holland GN, Helm CJ, et al. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. 1996; 121 (1): 35–46. DOI: 10.1016/s0002-9394(14)70532-xttp://www.reviewofoptome try.com/continuingeducation/tabviewtest/lessonid/107773. 6. Panova IE, Drozdova IE. [Uveitides: a manual for physicians]. Moscow: Meditsinskoie informatsionnoie agenstvo; 2014. Russian. 7. Nussenblatt RB, Whitcup SM, editors. Uveitis: fundamental and clinical practice. 4th ed. Elsevier/Mosby; 2012. 8. Emmett T Cunningham, Zierhut M. Uveitic Macular Edema. Ocul Immunol Inflamm. 2018;26(7):987–90, 9. Khramenko NI, Konovalova NV. Findings of ocular and brain hemodynamics in patients with anterior uveitis complicated by macular edema. J Ophthalmol (Ukraine). 2020; 4:14–22. 10. Panchenko NV, Samofalova MN, Gonchar EN, Litvishchenko AV, Friantseva MV. [Thinning of the peripapillary nerve fiber layer in uveitis complicated by optic nerve inflammation]. Archive of Ukrainian ophthalmology. 2016;3(1):50-3. Russian. 11. Penkov MA, Shpak NI, Avrushchenko NM. [Endogenous uveitis]. Kyiv: Zdorov’ia; 1979. Russian. 12. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140(3): 509– 516. 13. Deschenes J, Murray PI, Rao NA, Nussenblatt RB. & International Uveitis Study Group. International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008; 16: 1-2. 14. McNeil R. Grading of ocular inflammation in uveitis: an overview. Eye news. 2016; 22 (5): Fabruary/March; Available at: http://www.eyenews.uk.com. 15. Glanz S. [Biomedical statistics]. Moscow: Praktika;1998. Russian. 16. Cimino L, Auer C, Herbort CP. Sensitivity of indocyanine green angiography for the follow–up of active inflammatory choriocapillaropathies. Ocul Immunol Inflamm. 2000; 8(4): 275–83. 17. Ioyleva ЕЕ, Krivosheeva MS, Smirnova MA. [Unilateral optic disc edema: features of the differential diagnosis]. Tavricheskii medikobiologicheskii vestnik. 2013;(3):166-70. Russian. 18. Ioyleva E, Krivosheeva M. Microperimetry in the diagnosis of the first manifestation of optic neuritis in multiple sclerosis. J Neurol Sci. 2015; 357: 47. 19. Trusko B, Thort J, Jabs D et al. The Standardization of Uveitis Nomenclature (SUN) Project. Development of clinical evidence base utilizing informatics tools and techniques. Methods Inf Med. 2013; 7. 52 (3): 259–265. 20. Bennett JL. Optic Neuritis. Continuum (Minneap Minn). 2019; 25(5): 1236–64. 21. Shantha GJ, Crozier I, Hayek BR, Bruce BB, Gargu C, Brown J, Fankhauser J, Yeh S. Ophthalmic Manifestations and Causes of Vision Impairment in Ebola Virus Disease Survivors in Monrovia, Liberia. Ophthalmology. 2017; 124(2): 170–177. doi:10.1016/j.ophtha.2016.10.011.HHS Public Access. 22. Smit RL, Baarsma GS, de Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. J Int Ophthalmol. 1993; 17(2):71-76. 23. Beck RW, Cleary PA, Anderson MM et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med. 1992; 326(9): 581–8.
24. Whitley W, Sheppard J. The basics of uveitis. Rev Optom. 2011; Available at: http://www.reviewofoptometry.com/continuingeducation/tabviewtest/lessoni...
Conflict of Interest: Authors declare that there are no conflicts of interest that might influence their opinion on the subject matter or materials described or discussed in this manuscript.
|