J.ophthalmol.(Ukraine).2021;5:35-40.
|
http://doi.org/10.31288/oftalmolzh202153540 Received: 20 May 2021; Published on-line: 23 October 2021 Visual disturbances before and after transnasal endoscopic surgery for craniopharyngiomas K. S. Iegorova, A. A. Chukov; M. O. Guk, O. Ie. Skobska, L. V. Zadoianyi, M. R. Kostiuk The State Institution"Romodanov Neurosurgery Institute, National Academy of Medical Sciences of Ukraine"; Kyiv (Ukraine) TO CITE THIS ARTICLE: Iegorova KS, Chukov AA, Guk MO, Skobska OIe, Zadoianyi LV, Kostiuk MR. Visual disturbances before and after transnasal endoscopic surgery for craniopharyngiomas. J.ophthalmol.(Ukraine).2021;5:35-40. http://doi.org/10.31288/oftalmolzh202153540
Background: Craniopharyngiomas (CP) are benign epithelial tumors of the chiasmal and sellar region and/or the third ventricle region which arise from embryonic remnants of Rathke’s pouch. They are in close proximity to the surrounding nervous, endocrine and vascular structures of the brain such as the chiasm, optic nerves and tracts, hypothalamus, pituitary stalk, major vessels and their branches, including perforating branches of the anterior cerebral, posterior communicating, and posterior cerebral arteries. Tumor relation to the optic tracts is an important aspect of surgery for CP. The blood supply to the optic nerve/chiasm complex is usually not affected, which allows maintaining it perioperatively; however, sometimes in radical surgery for CP there is elimination of some perforating arteries from the blood supply and devascularization of the chiasm, causing ischemic abnormalities in the chiasm. Purpose: To determine the features of visual disturbances before and after surgery for craniopharyngioma. Material and Methods: We examined the medical records of a cohort of 61 patients who underwent treatment including transnasal endoscopic surgery for suprasellar craniopharyngioma at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2020. The main group included 48 patients (96 eyes). Of these, 46 patients had presented with visual disturbances (reduced visual acuity and/or visual field defects), and 2 patients exhibited them after, but not before surgery. Patients underwent clinical and neurological, eye, and otoneurological examination. Results: Visual disturbances in the presence of suprasellar craniopharyngiomas were found in 46 patients (75%), and their amount and severity substantially varied, which was associated with mechanical compression of the chiasm, growth of the tumor into the optic nerve and chiasm, as well as impaired chiasmal blood supply. The pupillomacular bundle and optic tracts are involved in exerting an effect on the posterior chiasm in suprasellar CP, which was reflected by the predominance of the symmetric chiasmal syndrome (39.1%) with bilateral optic atrophy, and accompanied by reduced visual acuity (78.3%), visual field defects (96.7%), bitemporal heteronymous hemianopia with central scotoma (27.2%), bitemporal paracentral scotoma (22.8%) and homonymous hemianopia (6.5%). The surgical treatment resulted in improvement or restoration of visual functions in 58.3% of patients. Postoperative worsening of visual functions was observed not only in patients who did exhibit visual disturbances preoperative (16.6%) but also in those who did not (4.2%), which was due to devascularization of the chiasm in radical surgery for CP. Conclusion: Transnasal endoscopic surgery for CP enables direct visualization and surgical control of the vessels supplying the chiasm. In addition, this approach allows performing a rather radical surgery with as low as 20.8% postoperative frequency of visual loss or appearance of visual disturbances. Keywords: skull-base tumors, craniopharyngioma, chiasmal syndrome, compressive optic neuropathy, chiasmal blood supply, devascularization of the chiasm, transnasal endoscopic surgery
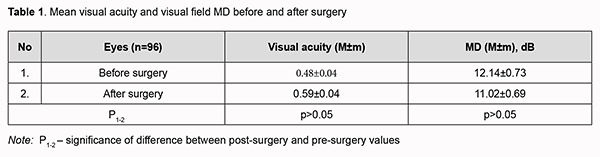
Introduction Craniopharyngiomas (CP) are benign epithelial tumors of the chiasmal and sellar region and/or the third ventricle region which arise from embryonic remnants of Rathke’s pouch [1, 2]. The clinical features of the disease depend on tumor location with respect to the pituitary stalk, chiasm, sella turcica diaphragm and third ventricle. Yassargil (1996) [3] divided craniopharyngiomas into a) purely intrasellar, infradiaphragmatic; b) intra- and suprasellar, infra- and supradiaphragmatic; c) supradiaphragmatic, parachiasmatic, extraventricular; d) intra- and extraventricular; e) paraventricular w/ respect to 3rd ventricle, and f) purely intraventricular. Because endosuprasellular craniopharyngiomas arise in the pituitary stalk, they can extend in various directions and cause the compression of the optic nerve/chiasm complex [1-4]. On a population scale, however, craniopharyngiomas are relatively rare lesions, with an incidence of only 0.17-0.2 per 100,000 person years. They comprise 0.8% of all intracranial neoplasms and constitute 13% of all suprasellular tumors. They are most common among children (0 to 14 years of age) and among older adults (aged 65-74 years), comprising 5-10% of all intracranial neoplasms and 56% of the sellar and suprasellar tumors in these age groups, although can occur in any age [5, 6]. Craniopharyngiomas are in close proximity to the surrounding nervous, endocrine and vascular structures of the brain such as the chiasm, optic nerves and tracts, hypothalamus, pituitary stalk, major vessels and their branches, including perforating branches of the anterior cerebral artery, posterior communicating artery, and posterior cerebral artery. The pathogenesis of visual disturbances is associated with the chiasmal compression by the cystic tumor component, devascularization of the optic chiasm, and direct tumor growth into the chiasm. Tumor relation to the optic tracts is an important aspect of surgery for craniopharyngioma. In the presence of a large tumor, the sites of the optic nerve/chiasm complex are compressed, expanded and dislocated. The blood supply to the optic nerve/chiasm complex is usually not affected, which allows maintaining it perioperatively; it is, however, sometimes difficult to distinguish the branches supplying the tumor from those supplying the visual pathways. The purpose of this study was to determine the features of visual disturbances before and after surgery for craniopharyngioma. Material and Methods We examined the medical records of a cohort of 61 patients (39 women and 22 men; age, 19 to 80 years; mean age, 50 ± 2.3 years) who underwent treatment including transnasal endoscopic surgery for suprasellar supradiaphragmatic craniopharyngioma at the Transsphenoidal Neurosurgery Department, Romodanov Neurosurgery Institute, during the period from 2017 through 2020. The aim of surgery was radical tumor removal (particularly, total or subtotal tumor removal in 57 cases, partial tumor removal in 2 cases, and tumor cyst aspiration in 2 cases). Patients underwent clinical and neurological, eye, and otoneurological examination (a routine otoneurological examination with assessment of cranial nerve function). Neuroimaging included T1-weighted magnetic resonance imaging (MRI) of the brain with a 1.5-T MRI system (Intera 1.5T/I system, Philips Medical Systems, Best, the Netherlands) and gadolinium-based intravenous contrast agent at a dose of 0.2 mL/kg body weight, and multispiral computed tomography. Neuro-ophthalmic examination included best-corrected visual acuity assessment, biomicroscopy, static automated and kinetic perimetry, and direct and indirect ophthalmoscopy. The first examination was performed on day 1 or 2 after hospitalization, and the second, on day 5, 6 or 7 (early postoperative examination). Best-corrected visual acuity was classified as normal (1.0 or better), mild impairment (0.7-0.9), moderate impairment (0.4-0.6), severe impairment (0.1-0.3), and very severe impairment (< 0.1). Static automated perimetry (SAP) was performed with the Centerfield 2 Perimeter (Oculus, Wetzlar, Germany) using the neurological 30-2 threshold test program and Neuro screening program. Visual field loss severity was classified as “no visual field loss” (Grade 0; normal visual field), mild visual field loss (Grade 1; MD, –2 dB to –4 dB), moderate visual field loss (Grade 2; MD, –4 dB to –12 dB), severe visual field loss (Grade 3; MD, –12 dB to –20 dB), and very severe visual field loss (Grade 4; MD, worse than –20 dB) [7]. The visual field loss was classified as very severe if it was not possible to assess visual fields due to the extremely poor visual function. A chiasmal syndrome was considered symmetric if both eyes had the same grade of visual field loss. In addition, a chiasmal syndrome was considered asymmetric if the difference between eyes in grade of visual field loss severity was 1, and it was considered markedly asymmetric if the difference was 2 or greater. Because in some patients tumor removal resulted in improved visual acuity but worsened visual fields, severity of visual function loss was assessed taking into consideration both visual acuity and visual field MD in both eyes: mild visual function loss (normal visual acuity; MD, –2 dB to –4 dB), moderate visual function loss (visual acuity better than 0.1 in both eyes; MD, –4 dB to –12 dB), and severe visual function loss (visual acuity worse than 0.1 in one or both eyes; MD, worse than –12 dB in one or both eyes). A two-fold improvement (for postoperative VA worse than 0.1) or improvement by 0.2 or more (for postoperative VA better than 0.1) in VA with at least a 15 percent visual field expansion, reduction in scotoma size, or any improvement in MD was considered a positive change in visual function. Each patient underwent an otoneurological examination preoperatively and postoperatively, which included anterior and posterior rhinoscopy, otoscopy and pharyngoscopy. During this examination, special attention was paid to the presence of any suppurative inflammation in the nasal cavity and paranasal sinuses, which was a major contraindication for the transnasal approach. In addition, the presence of any of the following was recorded: nasal septum deformity, bony spurs, hearing disorder, vestibular disorder, or neurological deficiency due to cranial nerve lesions. Postoperatively, patients were examined for the presence or absence of signs of nasal liquorrhea, and the nasal cavity was washed with saline. The study protocol conformed to the tenets of the Declaration of Helsinki, and was approved by the Bioethics Committee of the Romodanov Neurosurgery Institute. Written informed consent was signed by all patients. Results are presented as the mean and standard error of mean (M ± SD). Student’s unpaired t test was used to determine differences between independent groups. The level of significance p ≤ 0.05 was assumed. Results Forty-six patients (75%; 92 eyes) had presented with visual disturbances (reduced visual acuity and/or visual field defects), and 13 patients, without visual disturbances before transnasal endoscopic surgery for the removal of craniopharyngioma. We believe that this was due to the fact that suprasellary craniopharyngiomas produce posterior chiasmal compression leading to visual disturbances (reduced visual acuity and/or visual field defects). The duration of visual disturbances was two weeks to two years, with a gradual decrease in visual function. Preoperatively, 20 eyes (21.8%) showed normal VA (1.0); 14 eyes (15.2%), mild VA impairment (0.7 – 0.9); 13 eyes (14.1%), moderate VA impairment (0.4 – 0.6); 21 eyes (22.8%), severe VA impairment (0.1 – 0.3); 15 eyes (16.3%), very severe VA impairment (< 0.1); and 9 eyes (9.8%) were blind. All the 46 patients (89 eyes) with visual disturbances showed visual field defects. Temporal hemianopia with central scotoma was the commonest field defect (25 eyes; 27.2%), followed by central scotoma with temporal visual field loss (21 eyes; 22.8%), temporal hemianopia (either complete or partial) only (15 eyes; 16.3%), and residual visual field in the nasal inner quadrant (15 eyes; 16.3%). In addition, visual field was not measurable due to extremely low visual function in 7 (7.6%) eyes. No change in the visual field was noted in 3 (3.3%) eyes. Ocular motility disorders (OMD) were found in 3 patients. Of these patients, one had isolated unilateral CNIII (oculomotor nerve) palsy, and two had isolated unilateral CNVI (abducens nerve) palsy. Ophthalmoscopy found primary descending optic atrophy (OA) in 30 (65.2%) patients. Of these, 28 patients (56 eyes) exhibited bilateral OA, and 2 patients (2 eyes), unilateral OA. Total OA was found in 9 eyes, and partial OA, in 49 eyes. Symmetric chiasmal syndrome was most common (18 eyes; 39.1%), followed by asymmetric (13 eyes; 28.3%) and markedly asymmetric (15 eyes; 32.6%). Analysis of visual acuity loss and visual field loss in both eyes found that severe visual function loss was most common (32 patients; 69.6%), followed by moderate visual function loss (11 patients; 23.9%) and mild visual function loss (3 patients; 6.5%). Early after surgery, visual field disturbances appeared but normal visual acuity maintained in 2 patients (3.3%) of the 61 that underwent transnasal tumor removal. In addition, of the 46 patients with disturbed visual functions at baseline, 6 (13.0%) showed restoration of visual functions (visual acuity and visual fields in both eyes), 22 (47.8%) showed improvement in visual functions, 10 (21.7%) showed no change in visual functions, and 8 (17.4%) showed worsening of visual functions early after transnasal tumor removal. Table 1 shows mean visual acuity and MD of visual field in patients before and after surgery. Mean visual acuity and MD of visual field improved after treatment, but the difference was not significant (p > 0.05).
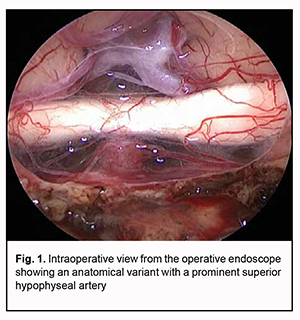
Visual acuity was still normal in 18 eyes (18.8%), restored to 1.0 in 17 eyes (17.7%), improved in 31 eyes (32.3%); did not improve in 22 eyes (22.9%) and worsened in 8 eyes (8.3%). Five eyes were still blind. Visual fields were still normal in 3 eyes (3.1%), became normal in 10 eyes (10.4%), improved in 36 eyes (37.5%), did not change in 26 eyes (27.1%) and worsened in 17 eyes (17.7%). In addition, visual field defects appeared in 4 eyes (4.2%). Visual field defects were found in 42 patients (83 eyes). Temporal hemianopia with central scotoma was the commonest field defect (23 eyes; 23.9%), followed by temporal hemianopia (either complete or partial) only (21 eyes; 21.9%), central scotoma with temporal visual field loss (13 eyes; 13.6%), residual visual field in the inner half (11 eyes; 11.5%), and homonymous hemianopia (10 eyes; 10.4%). In addition, visual field was not measurable due to extremely low visual function in 5 eyes (5.2%) and did not change in 13 eyes (13.5%). The number of patients with severe visual function loss decreased by 5 (15.6%), and visual functions were found to be restored in 6 patients (12.5%). Therefore, we found that the pupillomacular bundle and optic tracts are involved in exerting an effect on the posterior chiasm in suprasellar craniopharyngiomas, which is reflected by reduced visual acuity (78.3%), visual field defects (96.7%), bitemporal heteronymous hemianopia with central scotoma (27.2%), bitemporal paracentral scotoma (22.8%) and homonymous hemianopia (6.5%). Discussion The pathogenesis of visual disturbances in craniopharyngioma is intricate and is associated with mechanical compression of the chiasm (tumor mass effect), growth of the tumor into the optic nerve and chiasm, as well as impaired blood supply to the optic nerve/chiasm complex. That is why patients with craniopharyngiomas had various amounts of loss of visual acuity and/or visual fields; however, most commonly, they had symmetric chiasmal syndrome (39.1%) and bilateral optic atrophy (65.2%). The blood supply to the optic nerve/chiasm complex varies depending on the individual’s anatomy. Blood is supplied to the intracranial portion of the optic nerve and chiasm by the arteries arising from the ophthalmic, posterior communicating, and choroidal branches of the C4 segment of supraclinoid internal carotid artery (ICA) [8]. The hypophyseal and infundibular arteries play the most important roles. The superior hypophyseal arteries (SHA) are a group of one to five small branches that arise from the C4’s ophthalmic segment and terminate on the pituitary stalk and gland, but also send branches to the optic nerve and chiasm. The largest of the branches is often referred to as the SHA (Fig. 1).
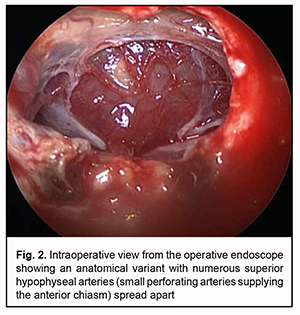
The infundibular arteries are a group of arteries that originate from the posterior communicating artery to the pituitary stalk. The SHA and infundibular arteries intermingle and form an anastomotic plexus called the circuminfundibular anastosmosis, and it is the small ascending arteries arising from the plexus that supply the inferior surface of the optic chiasm. Therefore, given the origin of craniopharyngioma growth (i.e., the pituitary stalk), it is impossible to imagine the growth of these tumors without the involvement of the above vascular formations into the pathological anatomy. In point of fact, circuminfundibular anastosmosis either extends around the whole surface of the enlarged pituitary stalk or becomes a portion of the capsule of craniopharyngioma. The SHA is frequently displaced anteriorly and laterally by the tumor, and, consequently, is very difficult to preserve during dissection of the tumor from the chiasm, especially if numerous small perforating arteries supplying the optic nerve/chiasm complex are spread apart. Devascularization of the chiasm should be understood as the exclusion of some perforating arteries of the SHA complex and circuminfundibular anastosmosis from the blood supply. Unfortunately, this is a common consequence of radical craniopharyngioma surgery. Most current publications on endoscopic surgery stress that it is a transnasal approach to the craniopharyngioma that enables better visual function outcomes, because only this approach enables direct visualization, surgical control and preservation of the perforating arteries of the anterior and inferior surface of the chiasm at the site of their contact with the tumor. In the current study, improvement or restoration of visual functions was observed in 58.3% of patients. Postoperative worsening of visual functions was observed not only in patients who did exhibit visual disturbances preoperative (16.6%) but also in those who did not (4.2%), which was undoubtedly due to devascularization of the chiasm. In has been reported that visual disturbances were found in 68.8%-73% (particularly, visual acuity disturbances, in 42%-44%, and visual field defects, in 55%-80%) of patients with supradiaphragmatic craniopharyngiomas [6, 9-12]. To our best knowledge, no detailed analysis of the outcomes of transnasal endoscopic surgery for craniopharyngioma in a large cohort of patients has been reported in the literature. Most studies, however, noted that endoscopic surgery was associated with lower incidence of trauma [1, 2, 5]. In the current study, visual function disturbances appeared or became more severe in 20.8%, whereas visual functions improved or restored in 58.3% of patients, which is in agreement with the current literature. In addition, 75% had presented with impaired vision, which is comparable with the data reported by Grković and colleagues and Koutourousiou and colleagues (73% and 68.8%, respectively). The probability of the presence of major visual acuity and/or visual field deficits after surgical excision of craniopharyngioma in the studies by Karavitaki and colleagues and Pereira and colleagues was 36% to 48% [6, 13]. Karavitaki and colleagues believe that the presence of visual impairment at the time of diagnosis increases the risk of vision loss after surgery [6]. Mortini and colleagues [5] conducted a meta-analysis on surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. The meta-analysis included 17 studies, with frequency of visual improvement ranging from 16% to 70%, and frequency of visual worsening, from 0% to 41%. Most authors are in agreement that the risk of visual worsening after gross total removal is substantially higher than after subtotal or partial removal of craniopharyngiomas, although the probability of visual improvement depends on the radicality of the surgical procedure [5, 6]. Intraoperative neurophysiologic monitoring is believed to be a promising option for preserving the optic nerve/chiasm complex in removal of craniopharyngiomas [14]. Therefore, we found that visual disturbances in craniopharyngioma substantially vary due to a complex interaction of several pathophysiological mechanisms. Assessment of the type and severity of ophthalmological impairments is a component of the assessment of (1) quality of the performed surgery, (2) adequacy of the selected surgical approach and (3) amount of justified craniopharyngioma resection. A large group of observations of current endoscopic radical treatment of craniopharyngioma was described, demonstrating the advantages of this approach given a relatively low percentage (20.8%) of patients with worsened visual functions. We believe that this was possible due to direct visualization and surgical control of the perforating arteries supplying the chiasm.
References 1.Cavallo LM, Frank G, Cappabianca P, Solari D, Mazzatenta D, Villa A, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121(1):100-13. 2.Kassam AB, Prevedello DM, Carrau RL, Snyderman CH, Thomas A, Gardner P, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg. 2011;114(6):1544–68. 3.Yasargil MG. Microsurgery Applied to Neurosurgery. Stuttgart: Thieme, 1969. 4.Louis DN, Ohgaki H, Wiestler OD, Webster KC, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. 5.Mortini P, Gagliardi F, Boari N, Losa M. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol. 2013 Dec;88(3):514-29. 6.Karavitaki N, Brufani C, Warner JT, Adams C, Richards P, Ansorge O, et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005 Apr;62(4):397-409. 7.Wall M, George D. Visual loss in pseudotumor cerebri: incidence and defects related to visual field strategy. Arch Neurol. 1987 Feb;44(2):170-5. 8.Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981 Oct;55(4):560-74. 9.Repka MX, Miller NR, Miller M. Visual outcome after surgical removal of craniopharyngiomas. Ophthalmology. 1989 Feb;96(2):195-9. 10.10. GrkovićD, Barisić S. Postoperative visual recovery following surgical treatment of craniopharygiomas. Med Pregl. Mar-Apr 2016;69(3-4):79-84. 11.Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Elizabeth C, Tyler-Kabara, Wang EW, et al. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(5):1194–207. 12.Jacobsen MF, Thomsen AS, Bach-Holm D, Doroudian G, Nissen KR, Fugleholm K, et al. Predictors of visual outcome in patients operated for craniopharyngioma - a Danish national study. Acta Ophthalmol. 2018 Feb;96(1):39-45. 13.Pereira AM, Schmid EM, Schutte PJ, Voormolen JH, Biermasz NR, Sjoerd W, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf). 2005 Feb;62(2):197-204. 14.Kim SM, Kim SH, Seo DW, Lee KW. Intraoperative Neurophysiologic Monitoring: Basic Principles and Recent Update. J Korean Med Sci. 2013; 28(9):1261–1269.
Conflict of Interest: The authors declare that there is no real or potential conflict of interest (financial, personal, professional or other interests) that could influence their opinion on the subject matter or materials described and discussed in this manuscript. Disclaimer: The views expressed in this article are those of the authors and not of the institution.
|