J.ophthalmol.(Ukraine).2021;5:28-34.
|
http://doi.org/10.31288/oftalmolzh202152834 Received: 15 July 2021; Published on-line: 23 October 2021 Ocular hemodynamics in patients with rhegmatogenous retinal detachment complicated by choroidal detachment N. I. Khramenko, M. M. Umanets, Z. A. Rozanova, G. V. Levytska SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: khramenkon@gmail.com TO CITE THIS ARTICLE: Khramenko NI, Umanets MM, Rozanova ZA, Levytska GV. Ocular hemodynamics in patients with rhegmatogenous retinal detachment complicated by choroidal detachment. J.ophthalmol.(Ukraine).2021;5:28-34. http://doi.org/10.31288/oftalmolzh202152834
Background: The occurrence of choroidal detachment (CD) in eyes with primary rhegmatogenous retinal detachment (RRD) is relatively uncommon (2%-8.6%). We hypothesized that significant disturbances in blood supply to the retina are a mechanism of the pathogenesis of RRD complicated by CD, which prompted us to study ocular hemodynamics in relevant patients. Purpose: To assess preoperative hemodynamics of the affected eye and fellow eye in patients with RRD complicated by CD. Material and Methods: We preoperatively examined the affected eye and fellow eye and assessed their hemodynamics in two groups of 11 patients each (the uncomplicated RRD group and the RRD complicated by CD group) which were similar with regard to age, time from the onset of RRD and ocular comorbidity. Results: Volumetric ocular pulse blood filling (OPBF) values in the affected eye and fellow eye of the latter group were 68.7% and 40%, respectively, lower, whereas of the former group, 33.3% and 27%, respectively, lower, than in age-matched normals. In addition, volumetric ocular pulse blood filling positively correlated with intraocular pressure (r=0.5; p < 0.05) in patients of both groups. This indicated that there is a significant ischemic process in the eye with RRD, which is aggravated in the presence of CD. Keywords: rhegmatogenous retinal detachment, choroidal detachment, ophthalmic rheography, ocular pulse blood filling, intraocular pressure
Introduction A rhegmatogenous retinal detachment (RRD) results from retinal breaks caused by vitreoretinal traction, with fluid from the vitreous cavity flowing through a retinal tear and dissecting underneath the retina. This allows fluid from the vitreous cavity to separate the sensory retina from the underlying retinal pigment epithelium, with the RPE pumping fluid from under the retina, and hypotony developing in the eye with RRD [1-4]. The occurrence of choroidal detachment (CD) in eyes with primary RRD is relatively uncommon (2%-8.6%) [5, 6]. High myopia, aphakia, pseudophakia, retinal detachment in three or more quadrants, advanced age and macular tear are major risk factors for developing a CD in patients with RRD [7, 8]. Clinical findings include red eye, pain of the globe with or without palpation, marked hypotony (5.0-8.0 mmHg), Descemet’s folds, deepened anterior chamber, corneal precipitates, posterior synechiae, iris color change, and sometimes iridophakodonesis. The vitreous appears hazy, and ophthalmoscopy shows a CD in the form of a prominent grey bubble extending from one to four quadrants, with the detached retina in the background. In 263 eyes with RRD complicated by CD, ranges of CD were <1 quadrant in 9 (3.42%) eyes, from 1 to 2 quadrants in 37 (14.07%) eyes, from 2 to 3 quadrants in 13 (4.94%) eyes, and from 3 to 4 quadrants in 204 (77.57%) eyes [9-11]. It has been hypothesized that, a more apparent disruption of the blood-retinal barrier and a more substantial release of inflammatory mediators take place in RRD complicated by CD compared to uncomplicated RRD, and consequently, a more severe disease course and worse anatomical and functional prognosis are seen in the former compared to the latter [12-14]. The separation of the photoreceptor layer from the RPE in RRD leads to an irreversible photoreceptor loss, with such pathophysiological mechanisms as apoptosis, necrosis, oxidative stress and inflammation underlying this process [15-19]. As a result, recovery of visual function after retinal reattachment will be only partial, especially if a detachment of the macular region is present. Postoperative period as well as visual recovery is more difficult in patients with RRD complicated by CD than in those with uncomplicated RRD. Xu and colleagues [20] used optical coherence tomography (OCT) angiography to observe changes in the blood flow in the macula in patients with RRD associated with CD (RRDCD) who underwent pars plana vitrectomy (PPV) for retinal reattachment and silicone oil filling, and found that the area of the deep plexus foveal avascular zone (DFAZ) continued to enlarge after the operation in the RRDCD group but did not significantly change in the RRD group. In addition, the correlation between BCVA at 3 months and DFAZ area one day after the operation in the RRDCD group was significant, and the authors concluded that choroidal lesions in RRDCD patients may have an acute pathological effect on ischemia of the deep retinal capillary network. We have previously demonstrated that, compared with patients with uncomplicated RRD, those with RRD complicated by CD exhibited lower bioelectrical activity of the central and peripheral retina after retinal reattachment post primary vitrectomy. We hypothesized that, in RRD complicated by CD, changes in the blood supply to and vascular changes in the ocular coats are more significant than in RRD only, which prompted us to study ocular hemodynamics in relevant patients [21, 22]. The purpose of the current study was to assess preoperative hemodynamics of the affected eye and fellow eye in patients with RRD complicated by CD. Material and Methods Twenty-two patients underwent an examination of the affected eye and fellow eye, and, of these, eleven patients (group 1; age, 53.5±3.5 years) underwent surgery for unilateral uncomplicated RRD, and the rest eleven (group 2; age, 60.6±3.3 years), for unilateral RRD complicated by CD, at the Vitreoretinal Surgery Department. Preoperatively, patients underwent a routine eye examination, assessment of electrical sensitivity of the optic nerve (electrically evoked phosphene threshold, EEPT) and critical frequency of phosphene disappearance (CFPD), and ophthalmic rheography (ORG). The latter study was performed with Reocom (KHAI-Medika, Kharkiv, Ukraine), the computerized rheography apparatus, with its operation based on the principle of impedancemetry. ORG included measurements of volumetric ocular pulse blood filling (OPBF, expressed as RQ, ‰ rheographic coefficient), vascular tone (expressed as alpha/T percentage index), and volumetric pulse blood filling rate (PBFR, expressed as Ohm/s). Preoperative treatment of eyes with RRD complicated by CD included intravitreal injection of 4-mg triamcinolone acetonide, and surgery for RRD was performed after either choroidal reattachment or a reduction in choroidal detachment height and extension was achieved. All eyes with either uncomplicated RRD or RRD complicated by CD received a 3-port 25-G vitrectomy. The retina was flattened with sterile air, subretinal fluid was drained through the primary retinal break. In addition, endolaser photocoagulation was performed to all retinal breaks, and, in some cases, 360-degree peripheral laser photocoagulation was performed. The surgical procedure was completed by endotamponade using a minimally expanding concentration of perfluoropropane. Statistical analysis was performed using Statistica 8.0 (StatSoft, Tulsa, OK, USA) software. Normally distributed data are presented as mean and standard deviation (SD) and non-normal data are presented as median and inter-quartile range (IQR). The Student's t test was used to determine significant differences between two normally distributed samples. Wilcoxon signed-rank test and Mann-Whitney U test were used for the comparison of two samples when the underlying distributions were not normal. Pearson’s correlation was used to test correlation between potentially dependent variables. Results Mean best-corrected visual acuity (BCVA) of affected eyes was 0.03±0.01, and fellow eyes, 0.97±0.02, for group 1 (uncomplicated RRD) versus 0.04±0.01 and 0.72±0.07, respectively, for group 2 (RRD complicated by CD). There was no significant difference in the visual acuity of affected eyes between groups, and visual acuity of fellow eyes was 25.8% higher in group 1 than in group 2 (p=0.02). Of the 11 affected eyes of group 1, five were emmetropic, two mildly myopic, two moderately myopic, and two highly myopic. Of the 11 affected eyes of group 2, three were emmetropic, three mildly myopic, three moderately myopic, and two moderately hypermetropic. Mean intraocular pressure (IOP) was 13.2±3.8 mmHg in group 1 vs 8.4±2.9 mmHg in group 2, and was 36.3% and significantly (p=0.02) higher in the former than in the latter. Of the patients of group 1, four (36.4%) had an IOL implanted vs five (45.4%) for group 2, and one eye in group 2 had vitreous hemorrhage. Of all the patients with RRD, 41% had an IOL implanted. Notably, no significant difference in the rate of ocular comorbidity was observed between groups. Among patients having an IOL implanted, the time after phacoemulsification ranged from 30 days to 7300 days. Of note that only for one patient the time after phacoemulsification was 30 days, whereas for the rest the time was longer than 180 days. The time from the onset of first symptoms to the diagnosis of retinal detachment ranged from 6 days to 30 days (mean value, 14.2 ± 3.1 days) for group 1 and from 10 days to 45 days (mean value, 17.8 ± 4.1 days) for group 2, i.e., the time of onset of RRD was practically the same for two groups. In group 1, in 2 eyes, the retinal detachment extended for two quadrants and in the rest eyes, for four quadrants. In all the eyes of group 2, the retinal detachment extended for four quadrants. No significant difference in the extent of retinal detachment in clock hours was observed between groups. The number or retinal breaks ranged from one to three or more. One retinal break was detected in 8 eyes, two breaks in one eye, three breaks in one eye, and more than three breaks in one eye in group 1, versus one break in four eyes, two breaks in one eye, three breaks in one eye, and more than three breaks in 5 eyes for group 2. More than three breaks were detected significantly more commonly (χ2 =3.7, p = 0.05) in patients with RRD complicated by CD than in patients with uncomplicated RRD (45.5% vs 9%). Our study of ocular hemodynamics found that volumetric ocular pulse blood filling (OPBF, expressed as RQ) in eyes with uncomplicated RRD (group 1) was 53% lower, and in eyes with RRD complicated by CD (group 2), 68.7%% lower than in age-matched normals, and these differences were statistically significant (p =0.0001) (Table 1). In addition, volumetric ocular pulse blood filling in eyes with RRD complicated by CD was 33% lower (p = 0.01) than in eyes with uncomplicated RRD (Table 1). Vascular tone in large-caliber vessels (expressed as alpha1/T percentage index) was elevated only in group 1, and was 25% higher than in age-matched normals (p=0.0001). In group 2, vascular tone in small-caliber vessels was 13% higher than in age-matched normals (p=0.01) (Table 1). We found the volumetric ocular blood filling rate in patients of group 1 and group 2 to be half of that in age-matched normals (p=0.0001; Table 1).
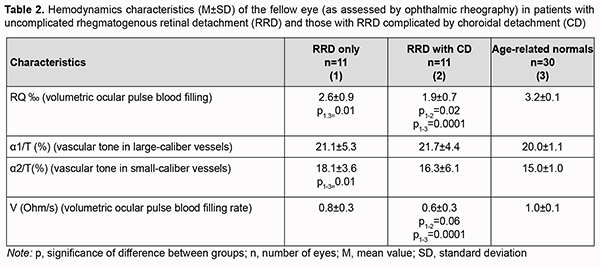
Of the fellow eyes of group 2, only three were healthy, three exhibited peripheral vitreoretinal degeneration; two, vitreochorioretinal degeneration; one had recent history of vitrectomy for RRD; one had degenerative myopia, and two had an IOL implanted. Of the fellow eyes of group 1, four were healthy, three exhibited vitreoretinal degeneration; three, vitreochorioretinal degeneration; and one had degenerative myopia. Volumetric ocular pulse blood filling in fellow eyes of patients of group 1 was 18.7% lower (p=0.01), and in fellow eyes of patients of group 2, 40% lower (p=0.0001) than in age-matched normals. The pairwise comparison of characteristics between patients with uncomplicated RRD and those with RRD complicated by CD showed that volumetric ocular pulse blood filling expressed as RQ was 27% lower for the latter than for the former group. In patients with uncomplicated RRD, vascular tone in small-caliber vessels in the fellow eye was 20% higher than in age-matched normals (p=0.01) (Table 2). We found that, in the fellow eye in patients of group 2, the OPBF rate was 40% lower than in age-matched normals (p=0.0001), and 25% lower than in the fellow eye in patients of group 1 (p=0.06) (Table 2).
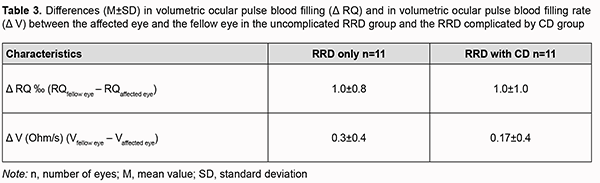
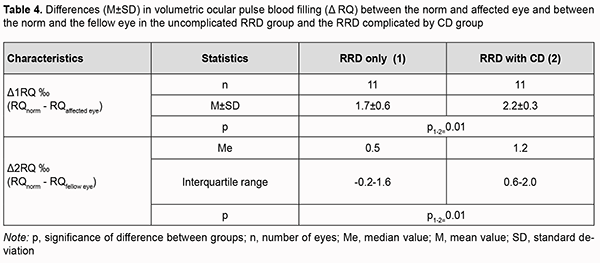
Therefore, ocular blood circulation was also impaired in the fellow eye of patients with RRD complicated by CD, with volumetric ocular pulse blood filling and OPBF rate being 40% lower than in age-matched normals. In addition, volumetric ocular pulse blood filling and OPBF rate in the fellow eye of patients with RRD complicated by CD were 27% (p=0.02) and 25% (p=0.06), respectively, lower than in the fellow eye of patients with uncomplicated RRD (Table 2). The differences in volumetric ocular pulse blood filling (Δ RQ) and in volumetric ocular pulse blood filling rate between the affected eye and the fellow eye (Δ V) were similar in both groups (Table 3). The differences in volumetric ocular pulse blood filling between the norm and the affected eye (Δ1RQ ‰) and between the norm and the fellow eye (Δ2RQ ‰) in group 2 were 23% larger (p = 0.01) and 2.4-fold larger (p = 0.01), respectively, than in group 1. This confirms that blood filling deficiency in the affected eye and the fellow eye was more severe in patients with RRD complicated by CD than in patients with uncomplicated RRD (Table 4).
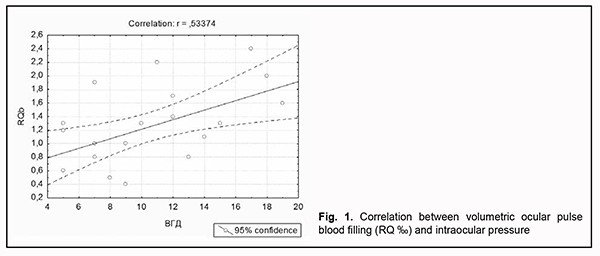
Volumetric ocular pulse blood filling positively correlated with IOP (r=0.5; p < 0.05) in patients with uncomplicated RRD as well as patients with RRD complicated by CD (Fig. 1).
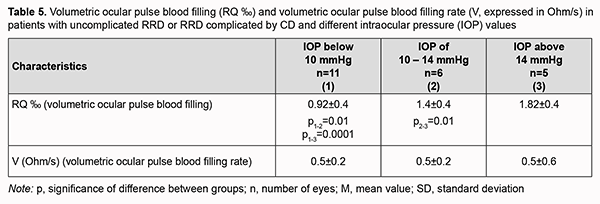
We identified three groups of study patients with IOP values below 10 mmHg (IOP_1 group, 11 eyes), 10-14 mmHg (IOP_2 group, 6 eyes), and above 14 mmHg (IOP_3 group, 5 eyes), to consider blood circulation in the affected eye in all patients with uncomplicated RRD or RRD complicated by CD and different IOP levels. We found that patients with IOP below 10 mmHg in both eyes had the lowest mean volumetric ocular pulse blood filling value (0.95 ± 0.4 ‰), which was 32% (p = 0.01) lower than in patients with IOP of 10-14 mmHg and 1.9-fold lower than in normal IOP (1.82 ± 0.4 ‰). Of note that volumetric ocular pulse blood filling (RQ) value in patients with IOP of 10-14 mmHg was 22.2% (p = 0.01) lower than in normal IOP (Table 5).
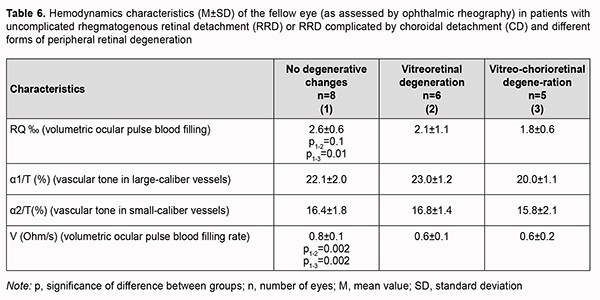
In patients with uncomplicated RRD or RRD complicated by CD, volumetric ocular pulse blood filling value in the fellow eye without focal fundus pathology was 2.6 ± 0.6‰, which was 18.7% (p = 0.0001) lower than the norm (Table 6). In addition, the mean volumetric ocular pulse blood filling value in the fellow eye with vitreoretinal degeneration or vitreochorioretinal degeneration was 2.0 ± 0.9‰, which was 37.5% (p = 0.0001) lower than the norm. Moreover, the mean volumetric ocular pulse blood filling value in the fellow eye with peripheral vitreochorioretinal degeneration was 31% (p = 0.01) lower than in the fellow eye without focal fundus pathology.
No significant difference in vascular tone was observed. Volumetric blood filling rate in the fellow eye without focal fundus pathology was 25% and significantly (p = 0.002) higher than in peripheral retinal degeneration. Discussion The two groups of patients included in this study (the group of patients with uncomplicated RRD and the group of patients RRD complicated by CD) were practically homogeneous with regard to age, time from the onset of RRD and ocular comorbidity. More than three breaks were detected significantly more commonly (χ2 =3.7, p = 0.05) in patients with RRD complicated by CD than in patients with uncomplicated RRD (45.5% vs 9%). This is in agreement with a study by Gu and colleagues [7] who found that, of the eyes with RRD complicated by CD, 34.62% presented with multiple holes (p = 0.044) and 25.00% with macular holes (p = 0.012), compared with 20.66% and 14.08% for eyes with RRD only. Of note that we failed to observe an association of CD with gender, age, time from the onset of RRD, and history of general medical conditions like hypertension. There are several works on blood supply disturbances in RRD complicated by CD, but they are mostly related to OCT angiography, i.e., structural changes in choroidal layers [20]. We found blood filling deficiency in the affected eye and the fellow eye both in patients with RRD complicated by CD and in patients with uncomplicated RRD. In addition, blood filling deficiency characteristics for the affected eye and the fellow eye in patients with RRD complicated by CD (68.7% and 40%) were twice as high as those in patients with uncomplicated RRD (33.3% and 27%, respectively). Moreover, we found the volumetric ocular blood filling rate in patients with RRD complicated by CD to be half of that in age-matched normals. Our findings demonstrate significant ocular blood supply disturbances in RRD in general, and especially in RRD complicated by CD. This also indicates that uncomplicated RRD or RRD complicated by CD is accompanied by significant hypoxia that affects not only the retina, but also other ocular coats, e.g., the choroid (particularly, the layer of large and middle choroidal vessels and the choriocapillaris layer). We believe that it is in agreement with a study by Xu and colleagues [20] who demonstrated that the area of the deep plexus foveal avascular zone continued to enlarge for three months after retinal reattachment in eyes with RRD complicated by CD. In addition, we found volumetric ocular pulse blood filling to be positively correlated with IOP (r=0.5; p < 0.05) in patients with uncomplicated RRD as well as those with RRD complicated by CD. A reduction in IOP in an uncomplicated retinal detachment is caused by increased RPE pumping. In an RRD complicated by CD, the above mechanism is joined by the hypotony associated with an inflammatory choroidal detachment and frequently inflammatory ciliary body detachment. It remains an open question whether marked hypotony precedes or follows choroidal detachment [23-25]. Therefore, the lower the IOP, the more apparent blood filling deficiency, or it may be vice versa (i.e., the more apparent ocular blood filling deficiency), and this creates a vicious circle of pathology that should be broken by means of surgical treatment as well as pharmacological correction. The differences in volumetric ocular pulse blood filling (Δ RQ) and in volumetric ocular pulse blood filling rate (Δ V) between the affected eye and the fellow eye were similar in the uncomplicated RRD group and the RRD complicated by CD group. What is important is that the volumetric ocular pulse blood filling value and the volumetric ocular pulse blood filling rate in the fellow eye with vitreoretinal degeneration or vitreochorioretinal degeneration were 37.5% lower than the norm and half of the norm, respectively. That is, it is likely that the state of the blood supply to ocular tissues (particularly, retinal and choroidal tissues) before severe structural injury in RRD complicated by CD occurs is important. This hypothesis requires further research, possibly involving studies on the state of brain vessels.
References 1.Foos RY, Wheeler NC. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982 Dec;89(12):1502-12. 2.Jaffe N.S. Complications of acute posterior vitreous detachment //Arch Ophthalmol. – 1968. – Vol.79. – P.568–571. Jaffe NS. Complications of acute posterior vitreous detachment. Arch Ophthalmol. 1968 May;79(5):568-71. 3.Tasman WS. Posterior vitreous detachment and peripheral retinal breaks. Trans Am Acad Ophthalmol Otolaryngol. Mar-Apr 1968;72(2):217-24. 4.Lindner B. Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol. 1966;87(suppl):1–108. 5.Sharma T, Gopal L, Reddy RK, et al. Primary vitrectomy for combined rhegmatogenous retinal detachment and choroidal detachment with or without oral corticosteroids: a pilot study. Retina. Feb-Mar 2005;25(2):152-7. 6.Yu Y, An M, Mo B, et al. Risk factors for choroidal detachment following rhegmatogenous retinal detachment in a Chinese population. BMC Ophthalmol. 2016 Aug 9;16:140. 7.Gu YH, Ke GJ, Wang L, et al. Risk factors of rhegmatogenous retinal detachment associated with choroidal detachment in Chinese patients. Int J Ophthalmol. 2016 Jul 18;9(7):989-93. 8.Kang JH, Kyung AP, Woo JS, Se WK. Macular Hole as a Risk Factor of Choroidal Detachment in Rhegmatogenous Retinal Detachment. Korean J Ophthalmol. 2008 Jun; 22(2): 100–3. 9.Li Z, Li Y, Huang X, et al. Quantitative analysis of rhegmatogenous retinal detachment associated with choroidal detachment in Chinese using UBM. Retina. Nov-Dec 2012;32(10):2020-5. 10.Jarrett 2nd WH. Rhegmatogenous retinal detachment complicated by severe intraocular inflammation, hypotony, and choroidal detachment. Trans Am Ophthalmol Soc. 1981; 79: 664-83. 11.de Smedt S, Sullivan P. Massive choroidal detachment masking overlying primary rhegmatogenous retinal detachment: A case series. Bull Soc Belge Ophtalmol. 2001;(282):51-5. 12.Sharma T, Gopal L, Badrinath SS. Primary vitrectomy for rhegmatogenous retinal detachment associated with choroidal detachment. Ophthalmology. 1998 Dec;105(12):2282–5. 13.Ghoraba HH. Primary vitrectomy for the management of rhegmatogenous retinal detachment associated with choroidal detachment. Graefes Arch Clin Exp Ophthalmol. 2001 Oct;239(10):733-6. 14.Dai Y, Wu Z, Sheng H, et al. Identification of inflammatory mediators in patients with rhegmatogenous retinal detachment associated with choroidal detachment. Mol Vis. 2015 Apr 10;21:417-27. 15.Cook B, Lewis GP, Fisher SK, Adler R. Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci. 1995 May;36(6):990-6. 16.Zacks DN, Hänninen V, Pantcheva M, et al. Caspase activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. – 2003 Mar;44(3):1262-7. 17.Trichonas G, Murakami Y, Thanos A, et al. Receptor interacting protein kinases mediate retinal detachment – induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci USA. 2010 Dec 14;107(50):21695-700. 18.Suzuki Y, Adachi K, Takahashi S, et al. Oxidative Stress in the Vitreous Fluid with Rhegmatogenous Retinal Detachment. J Clin Exp Ophthalmol. – 2017; 6:5. 19.Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009; 4(12):e8158. 20.Xu C, Wu J, Feng C. Changes in the postoperative foveal avascular zone in patients with rhegmatogenous retinal detachment associated with choroidal detachment. Int Ophthalmol. 2020 Oct;40(10):2535-43. 21.Аlibet Yassine, Ponomarchuk VS, Levytska GV, Khramenko NI. Comparing bioelectrical activity of the peripheral retina among myopic patients operated for rhegmatogenous retinal detachment complicated by choroidal detachment. J Ophthalmol (Ukraine). 2018;3:41–51. 22.Alibet Yassine, Ponomarchuk VS, Khramenko NI, Levytska GV. Comparing bioelectrical activity of the central retina among myopic patients operated for rhegmatogenous retinal detachment complicated by choroidal detachment. J Ophthalmol (Ukraine). 2018;4:7–25. 23.Dobbie JG. A study of the intraocular fluid dynamics in retinal detachment. Arch Ophthalmol. 1963 Feb;69:159-64. 24.Yoshioka H, Endo Y. Clinical observations on ocular tension in cases of retinal detachment. Rinsho Ganka. 1966;20:193–201. 25.Swan KG, Christensen L, Weisel JT. Choroidal detachment in the surgical treatment of retinal separation. AMA Arch Ophthalmol. 1956 Feb;55(2):240-5.
Conflict of Interest: The authors declare that there are no conflicts of interest that would influence their opinion on the subject matter or materials described and discussed in this manuscript.
|