J.ophthalmol.(Ukraine).2021;5:10-13.
|
http://doi.org/10.31288/oftalmolzh202151013 Received: 31May 2021; Published on-line: 23 October 2021 Monitoring MMP-2 and MMP-7 levels in traumatic wounds of the ocular adnexa O. V. Petrenko 1, M. M. Dranko 1, 2, V. V. Korniienko 3, L. V. Hrytsai 2 1 Shupyk National Healthcare University of Ukraine; Kyiv (Ukraine) 2 Sumy Regional Clinical Hospital; Sumy (Ukraine) 2 Medical Institute, Sumy State University; Sumy (Ukraine) E-mail: drankoma@ukr.net TO CITE THIS ARTICLE: Petrenko OV, Dranko MM, Korniienko VV, Hrytsai LV. Monitoring MMP-2 and MMP-7 levels in traumatic wounds of the ocular adnexa. J.ophthalmol.(Ukraine).2021;5:10-3. http://doi.org/10.31288/oftalmolzh202151013
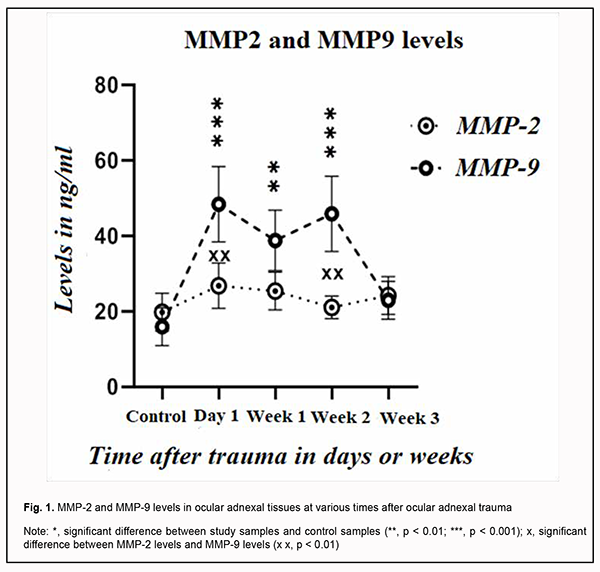
Background: Matrix metalloproteinase (MMP)-2 and MMP-9 are gelatinases involved in many physiological and pathological processes including inflammation and angiogenesis, which make up the basis for tissue remodeling and repair. Prolonged inflammation and delay in healing of traumatic wounds result in postoperative cicatricial deformities. MMP-2 and MMP-9 activity monitoring might be a biomarker for healing of traumatic ocular adnexal wounds. Purpose: The purpose of the study was to assess MMP-2 and -9 levels in tissues in traumatic ocular adnexal wounds at various times after trauma. Material and Methods: We examined 60 patients presenting with traumatic ocular adnexal wounds one hour to three months after trauma. Tissue biopsy was obtained during surgical debridement of wounds and frozen. The main study group included samples of traumatized ocular adnexal tissue, and the control group, samples of healthy ocular adnexal tissue obtained during blepharoplasty. The levels of MMP-2 and MMP-9 were assessed by enzyme-linked immunoassay (ELISA). The absorbance of each well was read at a wavelength of 450 nm with a Multiskan FC plate reader, and the mean absorbance was calculated for each reference standard and sample. Samples of patients presenting with traumatic ocular adnexal wounds one hour to three months after trauma were compared with controls with regard to optical density. Results: MMP-2 and MMP-9 levels were higher in biopsy samples from traumatic wounds than in control samples. ELISA analysis found that the peak MMP-2 expression was observed at day 3 after trauma, and was 85% above the level in control samples. From day 7 to day 15, this early peak fell to a level close to (15% higher than) that of day 1. From day 15 to day 21, there was a 35 percent increase in MMP-2 expression. MMP-9 expression levels were significantly higher than MMP-2 expression levels. The peak MMP-9 expression was observed at day 4 after trauma, and was 155% above the level in control samples. From day 4 to day 7, MMP-9 expression decreased to a level 85% above the level in control samples. Thereafter, MMP-9 expression increased again to a level 100% above the level in control samples from day 8 to day 15, and gradually decreased from day 15 to day 21. Conclusion: Our study of MMP-2 and -9 levels in tissues in traumatic ocular adnexal wounds at various times after trauma demonstrated that the peak MMP-2 expression was observed at day 3, and the peak MMP-9 expression, at day 4 after trauma. The levels of both enzymes decreased at day 7, and varied thereafter. By day 21, MMP-2 and MMP-9 levels in samples taken from the wound were as low as the levels in control samples. Keywords: MMP-2, MMP-9, traumatic wounds, wound healing, ocular adnexa, ELISA
Introduction Healing of traumatic wounds of the ocular adnexa is a complex mechanism with multiple processes orchestrating harmoniously for structural and functional restoration of the damaged tissue. Infected and chronic wounds with prolonged inflammation and delay in healing are a severe clinical complication because result in defective re-epithelization and undesirable postoperative scarring [1]. Matrix metalloproteinases (MMP) are major tissue remodeling and reparation enzymes, as they contribute to migration of fibroblasts and endothelial cells to the stroma, are actively involved in activation or deactivation of cell growth factors and biologically active molecules, regulate cell growth and apoptosis, leading to a balance between collagen synthesis and degradation [2-4]. Under normal physiological conditions, MMP levels and their activity are strictly controlled by many factors. MMP-2 and -9 are gelatinases capable of degrading amorphous collagen and fibronectin [2, 5, 6]. A clear understanding of the roles played by MMP-2 and -9 in the process of healing of ocular adnexal wounds, and a search for ways of controlling the complex interplay of these enzymes with extracellular matrix molecules may open new opportunities in the treatment of the infected and chronic wounds which frequently lead to functional and cosmetic as well as scarring complications. To the best of our knowledge, the literature is scarce with regard to the involvement of the MMPs in the wound process in the ocular adnexa, because the ocular globe (as well as its major components such as the anterior eye, media, ocular coats and optic nerve) has been the major focus of attention in studies on traumatic ocular injuries. This encouraged us to conduct a study on this subject. The purpose of the study was to assess MMP-2 and -9 levels in tissues in traumatic ocular adnexal wounds at various times after trauma. Material and Methods We examined 60 patients presenting with traumatic ocular adnexal wounds to an emergency room of the Department of Eye Microsurgery at the Sumy Regional Clinical Hospital one hour to three months after trauma. Wounds were classified by categories as traumatic infected and pyogenic, by the type of injury as incised, puncture, contused, lacerated, stub, concussion, bite, gunshot and mixed, and by the causative factor as mechanic (traumatic), chemical, thermal and trophic wounds. Patients were examined and treated according to the relevant care standards. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. The procedure for biopsy of traumatized tissues performed in the study involving human participants was in accordance with the ethical standards of the Ethical Committee (decision of the experts of the Ethical Committee of the Shupyk National Healthcare University of Ukraine, Protocol No. 1, Date of approval: 09.01.2020). Tissue biopsy was obtained during surgical debridement of traumatic wounds and frozen. The main study group included samples of traumatized ocular adnexal tissue, and the control group, samples of healthy ocular adnexal tissue obtained during blepharoplasty. 100-mg samples of traumatized ocular adnexal tissue were rinsed in phosphate buffered saline (PBS), homogenized in 1 mL of PBS, and left overnight at -20 °C. Two freeze-thaw cycles were applied to disrupt cell membranes, homogenates were centrifuged at 5,000 RPM for 5 minutes, and the supernatant was collected and sent for analysis. MMP-2 and MMP-9 level determination procedure was conducted at the Research Equipment Sharing Center of the Medical Institute at the Sumy State University. The levels of MMP-2 and MMP-9 were assessed by enzyme-linked immunoassay (ELISA) according to the manufacturers’ instructions using E-EL-HI1445, Human MMP-2 (Elabscience Biotechnology Co., Ltd) and BMS2016/2 Human MMP-9 (Affymetrix eBioscience, Bender MedSystems GmbH), respectively. The absorbance of each well was read at a wavelength of 450 nm with a Multiskan FC plate reader (Thermo Scientific, Waltham, MA, USA), and the mean absorbance was calculated for each reference standard and sample. A standard curve was created using Skanlt 4.1 microplate reader software capable of generating a 5-parameter logistic curve in order to determine the levels of MMP-2 and MMP-9 for each sample. The sensitivity of the method was 0.47 ng/ml for ММР-2 and 0.05 ng/ml for ММР-9. Samples of the 60 patients presenting with traumatic ocular adnexal wounds one hour to three months after trauma were compared with controls with regard to optical density. Statistical analysis was performed with SPSS 8.0 software (SPSS, Inc., Chicago, IL) using one-way analysis of variance to determine the significance of any difference between the estimates of the groups. Results are presented as the mean and standard deviation. The level of significance p ≤ 0.05 was assumed. Results MMP-2 and -9 are gelatinases involved in many physiological and pathological processes including inflammation and angiogenesis. The activity of these enzymes in the presence of wound process depends on the time from the traumatic event. We found a statistically significant increase in MMP-2 and -9 levels in the biopsy samples from traumatic wounds versus control samples. ELISA analysis found that the peak MMP-2 expression was observed at day 3 after the trauma, and was 85% above the level in control samples. From day 7 to day 15, this early peak fell to a level close to (15% higher than) that of day 1. From day 15 to day 21, there was a 35 percent increase in MMP-2 expression, likely due to proliferation of keratinocytes. MMP-9 expression levels were significantly higher than MMP-2 expression levels. The peak MMP-9 expression was observed at day 4 after the trauma, and was 155% above the level in control samples. From day 4 to day 7, MMP-9 expression decreased to a level 85% above the level in control samples. In addition, MMP-9 expression was as low as in controls from day 8 to day 15, and gradually decreased from day 15 to day 21. These variations were likely due to variations in the cell composition of traumatic wounds, because it is known that MMP-2 and MMP-9 enzymes are secreted by neutrophils, fibroblasts and macrophages. Subsequently, the gelatinases themselves regulate wound healing through effects exerted on inflammation, extracellular matrix remodeling and re-epithelization. We found that mean MMP-2 and MMP-2 levels changed with time after trauma (Fig. 1). MMP-9 expression was substantially higher than expression of MMP-2 and statistically significantly higher than expression of MMP-2 at day 1 and week 2 (p < 0.01). Peak expression levels of both MMP-2 and MMP-9 were observed early after trauma, and were 2.5-fold and 1.5-fold higher, respectively, than expression levels at day 1. A decrease in expression level of MMP-9 in study samples at week 1 was followed by a significant re-increase (p < 0.01) to a level twice the control values at week 2. However, the expression level of MMP-2 in study samples was increased (a peak expression level) within the first week, and decreased to almost the control level as early as week 2. Therefore, a gradual decrease in the expression levels of both MMPs to the same point was seen within three weeks.
Discussion Longitudinal changes in metalloproteinase levels in ocular adnexal tissues after trauma are indicative of their possible involvement in the wound healing process as a marker of inflammatory activity and degradation of the extracellular matrix as a result of tissue damage [6, 7]. MMP-2 and MMP-9 are involved in degradation of type IV collagen, a major component of the basement membrane. In addition, MMP-2 can degrade elastin and fibronectin by modulating protein functions and thus regulating the level of inflammation and causing vasoconstriction depending on its levels in tissues [8]. Variations in MMP levels can be explained by variations in the cell composition of traumatic wounds, because MMP-2 and MMP-9 enzymes are secreted by neutrophils, fibroblasts and macrophages. Subsequently, the gelatinases regulate wound healing through effects exerted on inflammation, extracellular matrix remodeling and re-epithelization [4]. Although increased MMP-2 and MMP-9 levels are associated with the level of inflammation and tissue damage, they accelerate tissue remodeling. Successive activation of the metalloproteinases is indicative of ocular tissue response to damage and triggering the pathogenetic mechanisms (MMP-9), and adaptation and adjustment to inhibition of local inflammation in the presence of extracellular matrix degradation (MMP -9) [3]. Conclusion Our study of the activity of MMP-2 and MMP-9 demonstrated that, for patients presenting with traumatic ocular adnexal wounds at different times after trauma and having a biopsy taken from the wound at presentation, peak MMP-2 expression was observed in biopsy samples taken at day 3, and peak MMP-9 expression, at day 4 after the traumatic event. The levels of both enzymes decreased at day 7, and varied thereafter. By day 21, MMP-2 and MMP-9 levels in samples taken from the wound were as low as the levels in control samples. Variations in MMP levels can be explained by variations in the cell composition between biopsy samples taken from traumatic wound tissues. Increased MMP-2 and MMP-9 levels are indicative of the activation of neoangiogenesis, which may be due to a shift in the proangiogenic balance in the presence of prolonged inflammation. In addition, increased levels of these enzymes indicate inflammation in traumatic wounds, and may be a marker of extracellular matrix remodeling. Understanding of the pathological phenomena associated with MMP-2 and MMP-9 during wound healing may provide an opportunity for regulation of tissue repair and finding new pharmacological treatment options for infected wounds and those failing to heal. References 1.Petrenko OV. [Clinical features and treatment of post-traumatic ocular adnexal defects]. Archive of Ukrainian ophthalmology. 2015;3(2):38-43. Ukrainian. 2.Petrenko OV, Bezrodnyi BG, Tykhomyrov AO. [Monitoring the course of wound process in pyogenic wounds]. Khirurgiia Ukrainy. 2014;2:65-9. Ukrainian. 3.Le NT. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007 Jan 1;12:1475-87. 4.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015. May-Jul 2015;44-46:113-21. 5.Petrenko OM, Tykhomyrov AO, Petrenko OV. [Changes in matrix metalloproteinase activity in chronic soft tissue wounds in diabetics treated with vacuum therapy]. Klinychna khirurgiia. 2016;6:58-60. Ukrainian. 6.Sokolov VA, Likhvantseva VG, Levanova ON, Nikiforov AA, Vygodin VA. [Matrix metalloproteinase expression in the tear fluid and complement factor H gene polymorphism in patients with open-angle glaucoma]. Meditsinskaia immunologiia. 2017;19(5):547-56. Russian. 7.Chamanga E. Effectively managing wound exudate. Br J Commun Nurs. 2015 Sep;Suppl Wound Care:S8, S10. doi: 10.12968/bjcn.2015.20.Sup9.S8. 8.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research. 2006 Feb 15;69(3):562-73. doi: 10.1016/j.cardiores.2005.12.002.
Acknowledgement: Center for Collective Use of Scientific Equipment of the Sumy State University Medical Institute Source of support: Center for Collective Use of Scientific Equipment of the Sumy State University Medical Institute. Conflict of Interest: We declare that we have no real or potential conflict of interest (financial, personal, professional, etc.) that could influence our opinion on the subject or materials described in this manuscript.
|