J.ophthalmol.(Ukraine).2021;5:84-87.
|
http://doi.org/10.31288/oftalmolzh202158487 Received: 22 March 2021; Published on-line: 23 October 2021 Chloroquine maculopathy in a patient on long-term chloroquine therapy for pulmonary sarcoidosis: a case report N. V. Malachkova, T. Ie. Kozlova National Pirogov Memorial Medical University, Vinnytsya, Optimal Vision Center; Vinnytsia (Ukraine) E-mail: malachkovanataliia@gmail.com TO CITE THIS ARTICLE: Malachkova NV, Kozlova TIe. Chloroquine maculopathy in a patient on long-term chloroquine therapy for pulmonary sarcoidosis: a case report. J.ophthalmol.(Ukraine).2021;5:84-7. http://doi.org/10.31288/oftalmolzh202158487 The article is on an important subject, chloroquine maculopathy associated with long-term use of chloroquine. Given that chloroquine has been widely used for the treatment of COVID-19, it is important to accumulate data on adverse reaction to chloroquine and its derivatives, especially in the populations of Europe where the practice of using these antimalarials was not so common as in Asia and Africa. The reported case exemplifies possible ocular side effects in patients on long-term use of chloroquine (Delagil). Key words: chloroquine maculopathy, case, diagnosis, treatment, mistakes
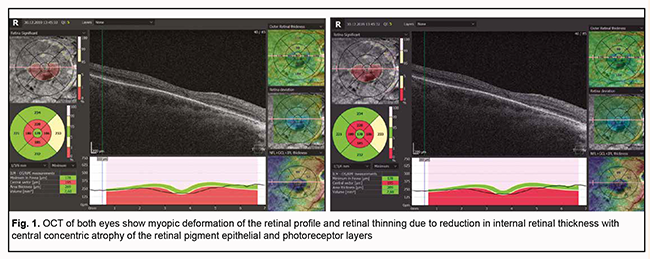
Introduction Antimalarial medications have been widely used in rheumatology primarily for the treatment of sarcoidosis, systemic lupus erythematosus, and rheumatoid arthritis for about 70 years. Chloroquine (CQ) is frequently recommended to be administered before hormonal treatment in early sarcoidosis. Sharma demonstrated that chloroquine is effective in controlling neurological sarcoidosis in those patients who fail to respond to corticosteroids or develop severe side effects. CQ and hydroxychloroquine (HCQ) exert an immune suppressive effect by influencing the metabolism of immunocompetent cells of the connective tissue [1]. Chloroquine phosphate was first synthesized in 1934 (sold under the brand name Delagil), and HCQ, in 1946 (sold under the brand name Plaquenil); both exert anti-inflammatory, anti-malaria, and mild immunosuppressive effects [2]. On April 2, 2020, the Health Ministry of Ukraine issued Order No.762, which approved the national protocol for the management of COVID-19 cases. Particularly, recommended doses and durations of treatment with CQ and HCQ were specified for various age groups and disease severity categories. However, a multinational, observational, real-world study of patients with COVID-19 found that the use of a regimen containing HCQ or CQ was associated with no evidence of benefit, but instead was associated with an increase in the risk of severe complications [3]. Given a dramatic increase in the number of patients receiving anti-malaria medications under conditions of the coronavirus pandemic and the probability of uncontrolled self-administration of these medications, our purpose was providing an increased awareness of physicians of a possible development of undesirable ocular effects. Clinical case In May, 2019, a 57-year-old female patient presented to the clinic and complained of seeing flashing lights and reduced near and distant vision. Her history was significant for pulmonary sarcoidosis and six-year therapy with Delagil at a dose of 6.5 mg/kg/day. She reported being myopic since childhood and used glasses. The patient’s uncorrected visual acuity (UCVA) OD was 0.06; the best-corrected visual acuity (BCVA) was 0.5 OD with a spherical correction of -7.0 D. The UCVA OS was 0.06; the BCVA OS was 0.5 OD with a spherical correction of -7.0 D and cylindrical correction of -0.75 D (40 degrees axis). Sphere, cylinder and axis values measured by noncyploplegic autorefractometry were 7.0 D, -0.5 D and 36°, respectively, OD, and 7.0 D, -1.0 D and 42°, respectively, OS. Icare intraocular pressure (IOP) was 14.0 mmHg OD and 13.0 mmHg OS. Axial length, anterior chamber depth and lens thickness were found to be 27.38 mm, 3.06 mm and 4.15 mm, respectively, for the right eye, and 27.42 mm, 3.21 mm and 4.01 mm, respectively, for the left eye. Amsler charts showed defects in the central visual field for both eyes. Status localis (the changes were symmetrical in both eyes). Anterior segment examination showed a bright cornea with individual punctal opacities within it, a moderately deep anterior chamber filled with clear aqueous, individual vacuoles within the lens, and pigment deposits on the anterior lens capsule. The vitreous showed mobile filamentous inclusions. Ophthalmoscopic examination showed pale pink optic discs, blurred foveal reflex, and annular hyperpigmentation in the central region. Optical coherence tomography found myopic deformation of the retinal profile and retinal thinning due to reduction in internal retinal thickness with central concentric atrophy of the retinal pigment epithelial (RPE) and photoreceptor layers (Fig. 1) in both eyes.
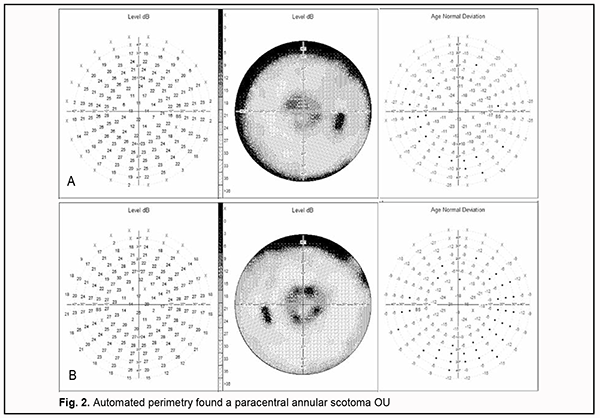
Automated perimetry found a paracentral annular scotoma OU (Fig. 2).
The patient was clinically diagnosed with chloroquine maculopathy, initial complicated cataract OU, high myopia with astigmatism OU, and paving stone peripheral retinal degeneration OU. She was referred to the pulmonologist and rheumatologist and it was recommended cessation of Delagil and to consider changing to another therapy for the underlying condition. The patient was prescribed a dietary supplement containing vitamins, intioxidant microelements, lutein, zeaxanthin, omega-3 fatty acids and red grape extraction. The supplement was designed to protect cells from the destructive effect of free radicals that accelerate aging and retinal cell destruction. In addition, the patient was recommended to receive the neuroprotective eye drops containing citicoline in order to restore the structure of damaged nerve cell membranes and protect cells from free radicals. Seven months after diagnosing CQ maculopathy, she reported no change in complaints. The patient received a consultation from the rheumatologist regarding her underlying disease. She was recommended cessation of Delagil and changing to Metypred, a corticosteroid. An insignificant decrease in visual acuity was noted, possibly due to cataract progression and myopization of the lens, which was confirmed by refractometry and optic biometry. The UCVA OD was 0.04, and the BCVA was 0.3 OD with a spherical correction of -8.50 D. The UCVA OS was 0.04, and the BCVA was 0.25 OD with a spherical correction of -9.50 D and cylindrical correction of -0.50 D (40 degrees axis). Sphere, cylinder and axis values measured by noncyploplegic autorefractometry were -8.75 D, -0.75 D and 39°, respectively, OD, and -9.75 D, -1.50 D and 45°, respectively, OS. Icare IOP was 12.0 mmHg OD and 14.0 mmHg OS. Axial length, anterior chamber depth and lens thickness were found to be 27.36 mm, 3.00 mm and 4.35 mm, respectively, for the right eye, and 27.43 mm, 3.07 mm and 4.42 mm, respectively, for the left eye. There was no change in OCT scans or automated perimetry data compared to month 7, which confirmed the sustainability of organic retinal changes even after cessation of the initial drug. In addition, lens thickness somewhat increased, from 4.15 mm to 4.35 mm OD and from 4.01 mm to 4.42 mm OS. Unfortunately, the patient has failed to save her ophthalmologist’s conclusion made prior to CQ therapy. However, the presence of ocular comorbidity confirmed the requirement for regular ocular screening of such patients, and this requirement was not fulfilled. Discussion In 2016, the American Academy of Ophthalmology published revised guidelines for screening and monitoring for CQ or HCQ retinopathy [4]. Included is a baseline ocular examination performed at the commencement of therapy. 1. No follow-up screening examination during the first 5 years of therapy is required if the results of a baseline ocular examination performed at the commencement of therapy were normal and if the daily dosage of HCQ utilized is ≤ 5 mg/kg/day actual body weight (or ≤2.3 mg/kg/day/ actual body weight for CQ). 2. If the dosage of hydroxychloroquine utilized is higher than 6.5 mg/kg/day (or >3 mg/kg/day for chloroquine), or if the patient is obese, has renal or liver dysfunction, has concomitant macular disease, or is more than 60 years of age, or if a total cumulative dose increased due to preliminary administration of CQ or HCQ, screening should be performed at least annually. The World Health Organization has put forth the definition of adverse drug reaction (ADR) as “any response to a drug which is noxious and unintended, and which occurs at doses used in man for prophylaxis, diagnosis or treatment” [5]. ● Type A is a dose-related ADR, features of which are that they are common, related to a pharmacological action of the drug and predictable. The risk of this type of reaction increases with an increase in the therapeutic dose and in case of low selectivity of drug effect and/or interaction with other drugs. ● Type B is an unpredictable ADR that can occur only in patients with increased sensitivity to the drug and associated with allergy or gene-related abnormality in enzyme systems. Examples of such reactions include drug intolerance, idiosyncratic reactions, allergic reactions and pseudo-allergic reactions. ● Type C ADR is chronic (long-term). The major advantage of antimalarial drugs used in rheumatology practice is a low frequency of undesirable effects [6]. Although CQ and HCQ are chemically similar, rheumatologists of various countries prefer the latter to the former, primarily due to a lower toxicity. Aviva-Zubieta and colleagues [7] compared the long term effectiveness between CQ and HCQ and found that the proportion of patients with side effects taking HCQ and CQ was 15% and 28%, respectively (p=0.001). Of note that the rate of side effects associated with antimalarial drugs significantly decreases with a reduction of the dosage of CQ utilized to <250 mg/day or 4 mg/kg (400 mg/kg or <6.5 mg/kg for HCQ) [8, 9]. The most common and mildest side effects are gastrointestinal side effects including nausea, stomach pain, and diarrhea (7-12%). Skin manifestations like urticaria, itching, and measles-like rash can develop in association with CQ (4.3-12%). However, gastrointestinal side effects and skin manifestations can rarely develop in association with HCQ (0-2% and 2-10%, respectively) [8, 9]. Central nervous system side effects are commonly mild and reversible (1.3% for HCQ and 12% for CQ); most common are headaches followed by light-headedness [9]. Other problems include tinnitus, insomnia and increased nervousness [8]. Retinal problems continue to be a major worry associated with use of antimalarial drugs in rheumatological practice. In a review of 647 patients treated with CQ for a mean of >10 years, and of 2043 patients treated with HCQ for a similar period, retinal toxicity was found to occur in 16 (2.5%) and 2 (0.1%) patients, respectively [8]. In 1957, Cambiaggi was the first to report on CQ retinopathy [10], and in 1967, Shearer and Dubois were the first to describe HCQ retinopathy. Ocular lesions induced by long-term use of antimalarials CQ or HCQ retinopathy is not the only ocular toxic effect induced by CQ derivatives, although the changes in the cornea, lens, and/or ciliary body are less common. Keratopathy (a form of drug-induced lipidosis) with subepithelial and intrastromal punctate deposits of non-metabolizable phospholipids can manifest as early as two to three weeks after the onset of administration of antimalarial drugs [11]. Epithelial and stromal edema can also develop. The pattern of corneal deposits can vary from diffuse punctate opacities to an aggregation of radial and whirling lines that converge and coalesce on a zone just beneath the centre of the cornea. It is noteworthy that these changes can reverse after cessation of the drug and infrequently result in reduced visual acuity, but patients may complain of halos around a light source and photophobia [11, 12]. Long-term administration of antimalarials rarely causes impaired accommodation and drug-induced ciliary muscle dysfunction is uncommon [11, 13]. Pathological changes in the lens with cataract development can also occur. Retinal lesions have the most unfavorable visual prognosis. Retinal lesions induced by long-term administration of CQ manifest similar to those induced by long-term administration of HCQ, but the risk of developing the former lesions is higher. They differentiate between chloroquine maculopathy (with lesions only in the macular area) and chloroquine pigment retinopathy, with lesions throughout the retina. Of note is that ocular lesions are bilateral in these cases. At presentation, patients complain of reduced vision, distorted images, difficulty in reading, color vision changes, light flashes, photofobia and loss of central vision. A loss of foveal light reflex is an early ophthalmoscopic finding in chloroquine maculopathy. Premaculopathy consists of fine pigmentary stippling of the macula and loss of foveal reflex. It may progress to true retinopathy that usually consists of stippled hyperpigmentation of the macula, and is surrounded first by a clear zone of depigmentation and then by a second ring of pigment, giving a bull’s eye appearance. Macular RPE atrophy develops in severe maculopathy. Peripheral pigment deposits and retinal vessel narrowing are a late disease finding [11, 14]. The triggering event for retinopathy was proposed to be accumulation of lipid complexes in both neuronal (ganglion and bipolar) and glial cells in the retina [15]. This results in DNA toxicity and necrotic cell death. Other suspected mechanisms include an increase in intracellular pH (leading to abnormal cell surface reception) and a decrease in prostaglandin synthesis. Conclusion The reported case exemplifies possible ocular side effects in patients on long-term use of chloroquine or its derivatives. We believe that a baseline ocular examination performed at the commencement of therapy as well as follow-up screening is a must for such patients. Retinal lesions have the most unfavorable visual prognosis. Retinal toxicity manifestations are more severe in patients on chloroquine (Delagil) therapy than in those on hydroxychloroquine therapy.
References
|