J.ophthalmol.(Ukraine).2021;5:64-70.
|
http://doi.org/10.31288/oftalmolzh202156470 Received: 05 March 2021; Published on-line: 23 October 2021 Neopterin level in the anterior segment of the eye in induced uveitis with ocular hypertension when treated by dipeptide carnosine I. M. Mykheitseva, N. V. Bondarenko, S. G. Kolomiichuk, N. B. Kuryltsiv SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) TO CITE THIS ARTICLE: Mykheitseva IM, Bondarenko NV, Kolomiichuk SG, Kuryltsiv NB. Neopterin level in the anterior segment of the eye in induced uveitis with ocular hypertension when treated by dipeptide carnosine. J.ophthalmol.(Ukraine).2021;5:64-70. http://doi.org/10.31288/oftalmolzh202156470
Background: We have previously demonstrated that the course of inflammatory process was significantly more severe in eyes with anterior uveitis with ocular hypertension (OHT) than in those with anterior uveitis with normal intraocular pressure (IOP), which was largely due to a more marked activation of enzymes producing active oxygen as well as peroxidative processes in uveal structures of experimental animals. Aside from oxidative stress characteristics, our attention has been drawn to neopterin as a potential marker of inflammation severity and the efficacy of inflammation control. Because there is a paucity of reports focused on the potential for using this characteristic as a diagnostic criterion in ocular disorders, especially in uveitis with raised IOP, studies on this subject are important. Purpose: To assess the impact of dipeptide carnosine on the level of neopterin in the rabbit’s anterior eye segment in anterior uveitis with ocular hypertension. Material and Methods: Forty-four Chinchilla rabbits (88 eyes) were divided into 4 experimental groups (group 1, 10 animals with induced OHT only; group 2, 10 animals with induced experimental non-infectious uveitis only; group 3, 12 animals with OHT induced prior to experimental non-infectious uveitis; group 4, 12 animals treated with carnosine for experimental uveitis with OHT). Rabbits of group 4 received 5% carnosine solution into the conjunctival sac, twice daily for the four weeks. The control group comprised 9 intact rabbits. Neopterin enzyme-linked immunosorbent assay kit was used to determine neopterin levels in uveal tract tissue, aqueous humor and tear samples according to the manufacturer’s instructions. Statistica 5.5 (StatSoft, Tulsa, OK, USA) software and parametric statistical tests were used for statistical analysis. Results: Neopterin levels in uveal tract tissue, aqueous humor and tear samples taken from rabbit’s eyes with induced OHT only were increased compared to controls but lower than those in non-infectious uveitis only. Neopterin levels in uveal tract tissue, aqueous humor and tear samples from rabbit’s eyes with induced both anterior uveitis and OHT (group 3) were 5.2-, 3.7- and 2.4 times higher, respectively, than in controls, and 42.5% (р < 0.05), 28.5% (р < 0.05) and 23.6% (р < 0.05) higher, respectively, than in samples taken from rabbit’s eyes of group 2. The carnosine treatment of induced both anterior uveitis and OHT (group 4) contributed to reduced levels of neopterin is samples under study, with neopterin levels in uveal tract tissue, aqueous humor and tear fluid being 39.4%, 38.9% and 30.2% lower, respectively, than in samples taken from rabbit’s eyes of group 3 (anterior uveitis plus OHT without treatment) (р < 0.01), and 3.2-, 2.2- and 1.7 times higher, respectively, than in controls (р < 0.001). Conclusion: High neopterin levels in the uveal tract tissues and anterior chamber aqueous fluid indicated its impact on the course of inflammation in animals with induced uveitis only and especially in those with induced both uveitis and OHT. A high neopterin level in the tear fluid in anterior uveitis can be considered a diagnostic marker of the severity of inflammation in the anterior segment of the eye. The carnosine treatment statistically significantly reduced neopterin levels in the uveal tract tissues, anterior chamber aqueous fluid and tear fluid in rabbit’s eyes with induced both uveitis and OHT. The changes in neopterin levels were caused by a reduction in the severity of inflammation in the anterior segment of the eye. Keywords: non-infectious uveitis, ocular hypertension, neopterin, carnosine, rabbits
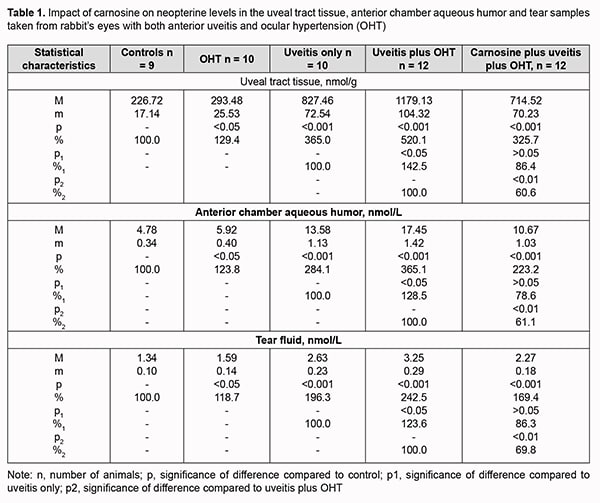
Introduction An increase in the prevalence of inflammatory anterior eye disease provides a significant medical and social challenge to our society [1, 2]. The disease most commonly occurs in young people and working-age adults, and some of its complications can cause a significantly impaired quality of life including blindness [3, 4]. Epidemiological studies have found that anterior uveitis is the most common type of intraocular inflammation and most commonly of noninfectious origin [5-7]. Raised intraocular pressure (IOP) has been attributed a major role in the development complications in degenerative and inflammatory processes in numerous publications on uveitis-associated conditions [8-10]. Oxidative stress characteristic particularly of glaucoma but also of uveitis, and significant oxidative balance disturbances contribute to the development of inflammatory processes in the presence of ocular hypertension (OHT) [11-14]. We have previously demonstrated that the course of inflammatory process was significantly more severe in eyes with anterior uveitis with OHT than in eyes with uveitis with normal IOP [15], which was largely due to a more marked activation of enzymes producing active oxygen as well as peroxidative processes in uveal structures of experimental animals [16]. Identification of characteristic biomarkers is essential for diagnostic procedures. Although a variety of characteristic biomarkers of inflammatory processes are already known, not all of them provide enough information. Excessive levels of oxidative stress components are pathogenic and contribute to an increase in inflammation, and not only they, but also significant changes in the cytokine and chemokine profile have been detected in the ocular tissues and biological fluids in uveitis [17, 18]. They, however, may not always be a reliable marker of inflammation in biological fluids (e.g., tear fluid) due to their short life span and paracrine effects [19, 20]. That is why our attention has been drawn to neopterin as a potential marker of inflammation severity and the efficacy of inflammation control. This compound is a stable low-molecular-weight heterocyclic product of monocytes, macrophages and other cells [20, 21], and regarded as a marker of activation of cellular immunity in a number of disorders. Given the role of immune mechanisms in the pathogenesis of uveitis, and that neopterin levels correlate with intraocular inflammatory response in an uveitis model [22, 23], neopterin level is a potential important marker of the severity of inflammation in uveitis [20, 24]. Because there is a paucity of reports focused on the potential for using this characteristic as a diagnostic criterion in ocular disorders, especially in uveitis with raised IOP, studies on this subject are important. It has been reported on increased levels of neopterin in uveitis of various etiologies and that these levels could correlate with the course of the disease [20, 25]. Presently available medications for the treatment of uveitis have significant side effects as well as a low efficacy to prevent complications of ocular inflammation, including ocular inflammation with ocular hypertension (OHT). The pathogenetic mechanisms of these conditions and a potential for an increase in efficacy-to–side effect ratio [26, 27] should be taken into account in order to reduce the rate and amount of the phenomenon. Because of this, in our studies we used the naturally occurring dipeptide carnosine (beta-alanyl-L-histidine), an anti-oxidant that has been shown to exhibit biological effects on the ocular structures in the treatment of various inflammatory and degenerative disorders [28], to assess its effect on the level of neopterin as a marker of the degree of inflammation. The purpose of this study was to assess the effect of dipeptide carnosine on the level of neopterin in the rabbit’s anterior eye segment in induced anterior uveitis with ocular hypertension. Material and Methods Forty-four Chinchilla rabbits (88 eyes) weighting 3.0 to 3.5 kg, maintained under normal vivarium conditions and fed and watered ad libitum, were used for all experiments. All animal experiments were performed in compliance with the General Ethical Principles of Animal Experiments (approved by Third National Congress on Bioethics, Ukraine, Kyiv, 2007) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes from the European Treaty Series (Strasbourg, 1986). Forty-four rabbits were divided into 4 experimental groups (group 1, 10 animals with induced OHT only; group 2, 10 animals with induced experimental non-infectious uveitis only; group 3, 12 animals with OHT induced prior to experimental non-infectious uveitis; group 4, 12 animals treated with carnosine for experimental uveitis with OHT). The control group comprised 9 intact rabbits. Non-infectious uveitis was induced by introducing bovine serum albumin at a dose of 5 mg into the conjunctival cavity only after the animal was sensitized [29]. OHT was induced by a single 0.1-mL injection of 0.3% carbomer into the anterior chamber of the rabbit. Carnosine was introduced by instilling 5% solution into the conjunctival sac of each eye in animals of group 4, twice daily for the four weeks. General anesthesia was administered by intramuscular injection of ketamine hydrochloride (50 mg/kg), and topical anesthesia was performed by instillation of 0.5% proparacaine hydrochloride into the conjunctival sac one minute before injection. Animals underwent biomicroscopy, ophthalmoscopy and tonometry. We used a Maklakoff tonometer with a 7.5-g plunger load to perform IOP measurements after topical anesthesia was performed by instillation of 0.5% proparacaine hydrochloride into the conjunctival sac. Uveal tract tissue, anterior chamber aqueous humor, and tear samples were harvested 4 weeks after completing the induction of non-infectious uveitis and OHT to determine neopterin levels. A 5 mm x 40 mm filter paper (Filtrak, Niederschlag Bärenstein, Germany) strip was folded and placed between the eyelid and the cornea and left for 1 minute. Thereafter, strip samples were eluted in physiological solution. The eluate was centrifuged (3,000 rev/min, 10 min) and the supernatant was collected for biochemical analysis. Animals were deeply anesthetized with thiopental sodium 10% (1.0 mL/kg, intramuscularly) and euthanized by air embolism. Eyes were enucleated and immediately placed on ice at 0 to 4 degrees C. A syringe with needle was used to harvest aqueous humor, and uveal tract tissue was separated from the sclera and optic nerve to prepare the homogenate. Neopterin levels were determined in the centrifuged supernatants using the neopterin enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions. Statistica 5.5 (StatSoft, Tulsa, OK, USA) software and parametric statistical tests were used for statistical analysis. Results Groups of animals with induced non-infectious uveitis only, induced OHT only, and induced both non-infectious uveitis and OHT varied in the degree of increase in the levels of neopterin, an inflammation marker. Neopterin levels in uveal tract tissue, aqueous humor and tear fluid from rabbit’s eyes with induced OHT only (group 1) were 29.4%, 23.8% and 18.7% higher, respectively, than in controls (Table 1).
The induction of anterior uveitis only resulted in a significant increase in neopterin levels in uveal tract tissue, aqueous humor and tear samples taken from rabbit’s eyes of group 2, by 265.0%, 184.1% and 96.3%, respectively, compared to controls (р < 0.001). Neopterin levels in uveal tract tissue, aqueous humor and tear fluid from rabbit’s eyes with induced both anterior uveitis and OHT (group 3) were 5.2-, 3.7- and 2.4 times higher, respectively, than in controls, and 42.5% (р < 0.05), 28.5% (р < 0.05) and 23.6% (р < 0.05) higher, respectively, than in samples taken from rabbit’s eyes of group 2. The carnosine treatment of induced both anterior uveitis and OHT (group 4) contributed to reduced levels of neopterin is samples under study, with neopterin levels in uveal tract tissue, aqueous humor and tear fluid being 39.4%, 38.9% and 30.2% lower, respectively, than in samples taken from rabbit’s eyes of group 3 (anterior uveitis plus OHT without treatment) (р < 0.01). In addition, although neopterin levels in uveal tract tissue, aqueous humor and tear fluid in eyes treated with carnosine for induced both anterior uveitis and OHT were significantly lower than in untreated eyes with induced both anterior uveitis and OHT, they were 3.2-, 2.2- and 1.7 times higher, respectively, than in controls (р < 0.001). Discussion We found increased neopterin levels in uveal tract tissue, aqueous humor and tear fluid of rabbit’s eyes with induced non-infectious uveitis. In addition, raised IOP contributed to an increase in inflammation marker levels. We have previously found an activating effect of OHT on oxidative stress [16] in the presence of an exhausted enzyme antioxidant system [30]. Oxidative stress activates an increased level of neopterin. A recent study [31] found that, compared with controls, neopterin concentration was increased in patients with pulmonary arterial hypertension, with an increase in the cytotoxic potential of activated macrophages and dendritic cells due to promotion of oxidative stress. Another recent study [32] found that serum interferon gamma, protein carbonyl, malondialdehyde and Neopterin levels were increased in infectious uveitis, and inflammation severity correlated with these markers. The authors noted that biomarkers in the aqueous humor could be a potential alternative for diagnosing a condition that may otherwise be thought to be idiopathic in uveitis. In the current study, we demonstrated increased neopterin levels in biological fluids of animal eyes with induced anterior uveitis. This may facilitate the development of potential diagnostic approaches and contribute to improved treatment efficacy. In addition, in the current study, neopterin levels were especially increased in animal’s uveal tract tissue. It has been demonstrated previously that periodontal diseases are characterized by enhanced macrophage infiltration to the periodontal lesion, so neopterin being a macrophage activation marker may be seen in higher levels [33]. A similar pattern could be observed in our data. Uveal tract tissues showed higher content of the inflammation marker than biological fluids, tear fluid and anterior chamber aqueous humor. However, somewhat increased neopterin levels were also seen in the group with induced OHT only. We believe this fact can be explained by the following mechanisms. Raised IOP facilitates the activation of oxidative stress processes and thus can induce inflammation, with a marker of the latter, neopterin, being increased in OHT. We noted these mild changes in the inflammatory status of the anterior eye segment in the group with induced OHT only. However, inflammation is accompanied by the activation of free radical oxidation that plays a major role in the pathogenesis of inflammation [34,35]. We have demonstrated previously [15,16,30] that ocular hypertension exacerbated the course of uveal tissue inflammation and worsened oxidative stress characteristics in uveitis. Our data on the use of dipeptide carnosine in rabbit’s eyes with induced uveitis and OHT demonstrate a significant anti-inflammatory effect of this substance in the anterior eye under conditions of induced comorbidities. We have reported previously [15] that carnosine facilitated significantly reduced pathological changes in ocular tissues in rabbit’s eyes with induced both non-infectious uveitis and OHT. A study on the anti-inflammatory effect of carnosine concluded that it is an effective adjunct in the treatment of keratitis and facilitated prompt re-epithelization [38]. Carnosine has been reported [36] to positively affect some age- and diabetes-related ocular diseases due to its metal-ion chelation and antioxidant capacity as well as the capacity to protect against formation of advanced glycation and lipoxidation end-products. Dipeptide carnosine, being an antioxidant, protects tissue cells from the pathogenic effect of oxidative stress and improves their resistance to increased functional loads caused by aging or pathological changes [37]. A positive effect of carnosine on healing of wounds of the cornea [39], lung tissue [40], and periodontium [41] has been demonstrated previously. In our experimental study, there was evidence of increased neopterin levels in the anterior chamber aqueous fluid, tear fluid and uveal tract tissues of animals with induced non-infectious anterior uveitis. Raised IOP facilitated increased levels of this inflammation marker in studies samples. High neopterin levels in the uveal tract tissues and anterior chamber aqueous fluid indicated the presence of a substantial oxidative stress as well as its impact on the course of inflammation in animals with induced uveitis only and especially in those with induced both uveitis and OHT. A high neopterin level in the tear fluid in anterior uveitis can be considered an effective diagnostic marker of the severity of inflammation in the anterior segment of the eye. Antioxidant carnosine treatment courses resulted in significant reductions in neopterin levels in the uveal tract tissues, anterior chamber aqueous fluid and tear fluid in rabbit’s eyes with induced both uveitis and OHT. The changes in neopterin levels in our experimental studies were caused by a reduction in the severity of inflammation in the anterior segment of the eye. We believe it reasonable to use the tear fluid neopterin level as a stable biomarker of the degree of inflammation in the anterior segment of the eye which permits for monitoring of the course of anterior uveitis and assessing the efficacy of therapy for the disease. The naturally occurring dipeptide carnosine, an anti-oxidant, can be recommended as an effective anti-inflammatory treatment option in anterior uveitis with OHT which reduces the level of neopterine, an inflammatory marker, in the uveal tract.
References 1. Katargina LA, Khvatova AV. [Endogenous uveitis in children and adolescents]. Moscow: Meditsina; 2000. Russian. 2. Gonzalez MM, Solano MM, Porco TC, et al. Epidemiology of uveitis in a US population-based study. J Ophthalmic Inflamm Infect. 2018 Apr 17;8(1):6. 3. Chang JH, Wakefield D. Uveitis: A global perspective. 2002 Dec;10(4):263-79. 4. Durrani OM, Meads CA, Murray PI. Uveitis: A Potentially Blinding Disease. Ophthalmologica. 2004;218:223-36. Crossref PubMed 5. Anesi SD, Foster CS. Anterior uveitis: etiology and treatment. Advanced Ocular Care. 2011;2:32-4. 6. Drozdova EA. [Issues of the classification and epidemiology of uveitis]. RMZh. Klinicheskaia Oftalmologiia. 2016;3:155-9. Russian. 7. Hosseini SM, Shoeibi N, Ebrahimi R, Ghasemi M. Patterns of Uveitis at a Tertiary Referral Center in Northeastern Iran. J Ophthalmic Vis Res. Apr-Jun 2018;13(2):138-43. 8. Aman R, Engelhard SB, Bajwa А, et al. Ocular hypertension and hypotony as determinates of outcomes in uveitis. Clin Ophthalmol. 2015 Dec 7;9: 2291-8. 9. Baneke AJ, Lim KS, Stanford М. The Pathogenesis of Raised Intraocular Pressure in Uveitis. Curr Eye Res. 2016;41(2):137-49. 10. Krasnozhan OV, Lutsenko NS, Zhaboiedov DG, Efimenko NF. [Features of the systemic expression profile of interleukins in patients with glaucoma and moderate myopia]. Oftalmologiia. Vostochnaia Evropa. 2019;4:471-80. 11. Ielskii VN, Mikheitseva IN. [Dysregulatory aspects of the glaucomatous process (review of the literature and own investigations]. Zhurnal NAMN Ukrainy. 2011;17(3):235-44. Russian. 12. Yadav UCS, Kalarya NM, Ramana KV. Emerging role of antioxidants in the protection of uveitis complications. Curr Med Chem. 2011;18(6):931-42. 13. Chesnokova NB, Neroev VV, Beznos OV, et al. [Oxidative stress in uveitis and its correction with superoxide dismutase antioxidative enzyme (experimental study)]. Vestn Oftalmol. Sep-Oct 2014;130(5):30-4. 14. Sunil SA, Wei J, Qian J, et al. Aqueous humor protein dysregulation in primary angle-closure glaucoma. Int Ophthalmol. 2019;39(4):861-71. 15. Mykheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. Clinical picture of uveitis in the presence of ocular hypertension and improvement in disease course with dipeptide carnosine. J Ophthalmol (Ukraine). 2021; 1:55-61. 16. Mykheitseva IM, Bondarenko NV, Kolomiichuk SG, Siroshtanenko TI. State of oxidation and peroxidation in rabbit’s uveal tract tissues in induced uveitis and ocular hypertension. J Ophthalmol (Ukraine). 2019;2:55-60. 17. Ooi KGJ, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis – is there a correlation with clinical phenotype? Clin Med Res. 2006 Dec;4(4):294-309. 18. Takase H, Sugita S, Taguchi С, et al. Capacity of ocular infiltrating T helper type 1 cells of patients with non-infectious uveitis to produce chemokines. Br J Ophthalmol. 2006 Jun;90(6):765-8. 19. Karaulov AV, Kaliuzhin OV. [Cytokines: biological action and clinical applications]. Uspekhi klinicheskoi immunologii i allergologii. Moscow: RAEN. 200;1:193-205. Russian. 20. Dikinov ZKh. [Molecular markers of inflammation in endogenous uveitis]. Immunopatologiia, allergologiia, infektologiia. 2013;3:22-26. Russian. 21. Sviridov EA, Telegina TA. [Neopterin and its reduced forms: biological role and participation in cellular immunity]. Uspekhi biologicheskoi khimii. 2005;45:355-390. Russian. 22. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120(9):3073-83. 23. Serif N, Gurelik G, Hasanreisoglu M, et al. Evaluation of Neopterin Levels in an Endotoxin-Induced Experimental Uveitis Model. Semin Ophthalmol. 2016;31(3):256-60. 24. Onishchenko AL, Kolbasko AV, Chernysheva AD, Surzhikova GS. [The value of neopterin in etiological diagnosis of endogenous uveitis]. Vestn Oftalmol. Mar-Apr 2011;127(2):25-9. Russian. 25. Palabiyik SS, Keles S, Girgin G, еt al. Neopterin Release and Tryptophan Degradation in Patients with Uveitis. Current Eye Res. 2016;41(11):1513-7. 26. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008 Aug;17(5):350-5. 27. Krakhmaleva DA, Pivin EA, Trufanov SV, Malozhen SA. [Modern opportunities in uveitis treatment] Ophthalmology in Russia. 2017;14(2):113-119. (In Russ.) 28. Volkov OA. [Biological role of carnosine and its use in ophthalmology (mini-review)]. Biomed Khim. Sep-Oct 2005;51(5):481-4. 29. Information Bulletin No. 19 issued 10.10.2019, based on Pat. of Ukraine №137,107; MPK (2019.01) А61К 9/00. [Method for inducing non-infectious uveitis in the presence of ocular hypertension]. Authors: Mykheitseva IM, Kolomiichuk SG, Bondarenko NV, Siroshtanenko TI. Owner: State Institution Filatov Institute of Eye Diseases and Tissue Therapy, NAMS of Ukraine. 30. Mykheitseva IM, Bondarenko NV, Kolomiichuk SG, et al. The effect of high intraocular pressure on enzymatic antioxidant system of uveal tissues in rabbits with experimental allergic uveitis. J Ophthalmol (Ukraine). 2019;4:57-63. 31. Smukowska-Gorynia A, Marcinkowska J, Chmara E, et al. Neopterin as a biomarker in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respiration. 2018;96(3):222–30. 32. Kashyap В, Goyal N, Das GK, et al. Ophthalmic Presentation of Disseminated Tuberculosis with Relapse-Immunological Profile. Ind J Clin Biochem. 2018 Oct;33(4):483-6. 33. Prasanna JS, Sumadhura C, Karunakar P. Neopterin as a diagnostic biomarker for diagnosis of inflammatory diseases like periodontitis. J Oral Res Rev. 2017;9:45-9. 34. Zenkov NK, Lankin VZ, Menshchikova EB. [Oxidative stress: Biochemical and pathophysiological aspects]. Moscow: MAIK Nauka/Interperiodica; 2001. Russian. 35. Khurtsilava OG, Pluzhnikov NN, Nakatis IaA, editors. [Oxidative stress and inflammation: a pathogenetic partnership]. St Petersburg: Mechnikov North Western State Medical University Publishing House; 2012. Russian. 36. Maichuk IuF, Kurenkov VV, Maichuk DIu, et al. [Efficacy of carnosine eye drops in corneal therapy and excimer laser surgery]. Oftalmol Zh. 2000;4:24-5. Russian. 37. Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013 Oct;93(4):1803-45. 38. Boldyrev AA, Stvolinsky SL, Fedorova TN. [Carnosine: endogenous physiological corrector of antioxidative system activity]. Usp Fiziol Nauk. Jul-Sep 2007;38(3):57-71. 39. Shekhter AB, Silaieva SA, Aboiants RK, et al. [A method of wound treatment]. Certificate of authorship No. 491184/14, USSR; 1991. Russian. 40. Perelman MI, Kornilova ZKh, Paukov VS, et al. [Impact of carnosine on lung wound healing]. Biulleten eksperimentalnoi i biologicheskoi meditsiny. 1989;108(9):352-5. Russian. 41. Seleznev DA. [Use of carnosine for the prevention and treatment of periodontal inflammatory disease in orthopedic treatment]. Thesis for the Degree of Cand Sc (Med). Moscow;2006. Russian.
Conflict of Interest: Authors declare that there are no conflicts of interest that might influence their opinion on the subject matter or materials described or discussed in this manuscript.
|