J.ophthalmol.(Ukraine).2021;4:48-52.
|
http://doi.org/10.31288/oftalmolzh202144852 Received: 08 April 2021; Published on-line: 16 August 2021 Role of cytokines, IL1-β and IL-10, in the development of endocrine ophthalmopathy in Graves’ disease Yu. V. Buldygina, H. A. Zamotaieva, G. M. Terekhova, N. M. Stepura, V. M. Klochkova, T. V. Fed’ko SI “V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine”; Kyiv (Ukraine) TO CITE THIS ARTICLE: Buldygina YuV, Zamotaieva HA, Terekhova GM, Stepura NM, Klochkova VM, Fed’ko TV. Role of cytokines, IL1-β and IL-10, in the development of endocrine ophthalmopathy in Graves’ disease. J.ophthalmol.(Ukraine). 2021;4:48-52. http://doi.org/10.31288/oftalmolzh202144852 Background: Endocrine ophthalmopathy (EO) is a disease which is characterized by progressive autoimmune inflammation of extraocular muscles and retrobulbar adipose tissue, and is accompanied by infiltration, edema and proliferation of retrobulbar adipose tissue, muscles and connective tissue, leading to worsened quality of life, limited capacity for work and even ocular atrophy. It has been demonstrated that cytokines are involved in the development of autoimmune ophthalmopathy in Graves’ disease (GD). Thus, the secretion of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and TNFα were increased several dozenfold in active ophthalmopathy, but the specificity of cytokines in EO is still a subject of discussion among researchers. Purpose: To assess the levels of proinflammtory cytokines (IL-1β) and anti-inflammatory cytokines (IL-10) in Graves’ disease complicated by endocrine ophthalmopathy. Material and Methods: Forty-three patients with Graves’ disease (33 women and 10 men; age, 18 to 71 years) underwent an examination. They were divided into two groups based on the presence or absence of EO: GD plus EO (24 patients) and GD only (19 patients). Disease duration was 2.2±0.4 years for patients with GD plus EO versus 3.98±0.74 years for patients with GD only (р<0.05). All patients were treated with antithyroid drugs (mercazolil and thyrozol) and were classified as having compensated thyroid disease at the time of the study. All patients with GD plus AO had a CAS score exceeding 3 (the active stage of EO). Patients underwent an examination including ultrasonic assessment of thyroid gland volume, hormonal examination (serum TSH, free T4 (fT4), and free T3 (fT3)), assessment of serum antithyroid antibodies (anti-thyroid peroxidase antibodies and TSHR-Ab) and serum IL-1β and IL-10 levels. Results: Serum IL-1β levels were significantly increased in patients with Graves’ disease compared to healthy controls. Serum IL-1β levels for patients with GD plus EO were significantly higher than for patients with GD only (45.48 ± 16.19 pg/ml versus 10.44±5.17 pg/ml; р < 0.05), which may indicate the specificity of this cytokine as a marker of inflammatory autoimmune activity in the orbit. Serum IL-10 levels were significantly increased in all patients with Graves’ disease, with no significant difference in this characteristic between patients with GD plus EO and patients with GD only (23.76±7.72 pg/ml versus 22.21±2.82 pg/ml; р > 0.05). Keywords: Graves’ disease, endocrine ophthalmopathy, IL-1β, IL-10
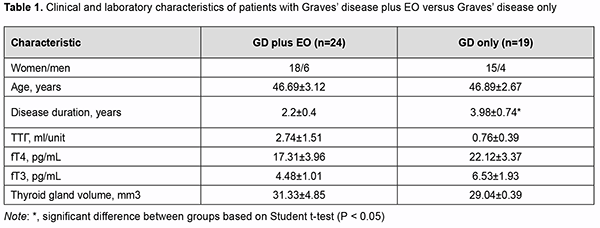
Introduction Endocrine ophthalmopathy (EO) is a disease which is characterized by progressive autoimmune inflammation of extraocular muscles and retrobulbar adipose tissue, and is accompanied by infiltration, edema and proliferation of retrobulbar adipose tissue, muscles and connective tissue, leading to worsened quality of life, limited capacity for work and even ocular atrophy. The role of cytokines in the pathogenesis of autoimmune disease, particularly, EO, has been extensively studied in the recent decade. Cytokines are endogeneous peptides with a molecular mass of 5-30 kD and are produced by various immune cells, endothelial cells, fibroblasts, etc. [1]. The effects of proinflammatory cytokines (e.g., interleukin (IL)-1β) are primarily associated with increased syn-thesis of acute proteins, pyrogens, prostaglandins, and collagen, and their capacity to affect vascular endothelium [2, 3]. IL-1 is produced by numerous cells, including activated macrophages and neutrophils, stimulated B-cells, and fibroblasts. At present, the IL-1 family comprises a total of 11 members. The most important, IL-1α and IL-1β, are coded by different genes, but similar with regard to structure, biological activity, and the receptors they connect to. IL-1α activates mostly T-cells and acts in an autocrine or paracrine manner, whereas IL-1β is a multifunctional cytokine with a diverse range of intra- and extra-cellular activities [2]. IL-1β secretion is part of inflammatory response in numerous types of immune and non-immune cells. IL-1β is secreted as a precursor protein, whereas IL-1α is constitutively expressed in numerous types of immune cells, usual-ly expressed as an intracellular precursor protein or can function as a membrane-bound cytokine [4]. IL-1β is pleiotropic in biological activity and is involved in the regulation of immune inflammatory response and immune response. It stimulates the antigen-presenting function of macrophages, activates a specific subpopulation of immune cells (innate lymphoid cells (ILC)3) and extracellular membrane proteins, is involved in the formation of post-infection immunity, inhibits proteoglycan and collagen synthesis, stimulates differentiation of osteoclasts from the mononuclear precursors leading to bone resorption. Like other proinflammatory mediators, IL-1β is involved in the formation of the general symptoms (like fever, loss of appetite, social disadaptation, etc.) of inflammatory dis-ease [5, 6, 7, 8, 9]. The diseases with hyperproduction of IL-1β (particularly, congenital autoimmune diseases, gout, Still's disease, Schnitzler syndrome, Behcet's disease, neutrophil dermatoses, rheumatoid arthritis, etc.) are characterized by the development of severe inflammation [2]. IL-10 is a proinflammatory cytokine that inhibits inflammation and cytokine cascade [10], and is produced mostly by monocytes and to a less extent by lymphocytes, including Th2 cells, mast and Treg cells, and some sub-populations of activated T and B cells. It is a pleiotropic cytokine that plays an important role in the regulation of functions of lymphoid and myeloid cells. The IL-10 is a potent inhibitor of cytokine synthesis and cell functions of macrophages, and suppresses effector functions of macrophages, T cells and NK cells. In addition, it is involved in the proliferation and differentiation of B cells, mast cells and thymocytes [11]. Moreover, it inhibits the proliferative response of T cells to antigens and mitogens, and alters the capacity of monocytes to present antigens to T-cells and to produce cytokines (IL-1, IL-6, IL-8, IL-12, tumor necrosis factor (TNF)α, GM-CSF, G-CSF) [13]. The activation of IL-10 production and a shift in the Th1/Th2 balance towards Th2 cells may be due to the ef-fect exerted by endotoxin or the release of catecholamines and glucocorticoids as inflammatory response to the stress induced by bacterial or chemical factors. Therefore, IL-10 is considered as a possible key factor leading to the inhibition of inflammation and initiation of regenerative processes. It has been demonstrated that cytokines are involved in the development of autoimmune ophthalmopathy in Graves’ disease. Thus, the secretion of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, and TNFα were increased several dozenfold in active ophthalmopathy [12]. The specificity of cytokines in EO is a subject of discussion among re-searchers. Thus, many believe that any of the currently known interleukins is not specific for EO [13]. It has been, however, reported on the pathogenetic role of IL-6 which increases the expression of thyroid-stimulating hormone (TSH) receptor in orbital fibroblasts and specific antibody synthesis by B cells, with CD8+CD28-lymphocytes in peripheral blood and serum concentrations of soluble IL-6 receptor correlating with disease activity [14]. It has been demonstrated recently that, in the active phase of EO, IL-17 was involved in the pathologic activa-tion of orbital fibroblasts (CD90), preadipocytes, and, in this way, contributed to orbital adipogenesis [15]. In addi-tion, IL-17 levels correlated directly with TSH-R antibody levels, laboratory markers of autoimmune activity in EO [15]. IL-4 causes fibroblast proliferation and contributes to hyaluronic acid production in the late stage of EO, con-tributing to the development of fibrosis in retrobulbar adipose tissue [16]. Elevated levels of proinflammatory cyto-kines, IL-4 and IL-10, have been reported in the active stage of EO and in a more severe course of the disease [12]. Therefore, further research on the role of cytokines in the onset and progression of EO is important and will have a practical value in the search for laboratory markers of inflammation in this pathology. Summing up the above data, studies on cytokine-mediated pathway for the development of endocrine oph-thalmopathy in Graves’ disease will be important for the development of novel therapeutic treatment strategies. The purpose of the study was to assess the levels of proinflammtory cytokines (IL-1β) and anti-inflammatory cytokines (IL-10) in Graves’ disease complicated by endocrine ophthalmopathy. Material and Methods Forty-three patients with Graves’ disease (33 women and 10 men; age, 18 to 71 years) underwent an examina-tion. They were divided into two groups based on the presence or absence of autoimmune ophthalmopathy (AO): GD plus AO (24 patients; 18 women and 6 men; age, 18 to 71 years; mean age, 46.69±3.12 years) and GD only (19 patients; 18 women and 6 men; age, 27 to 67 years; mean age, 46.89±2.67 years). Patients underwent an examination including ultrasonic assessment of thyroid gland structure, hormonal exam-ination (TSH, free T4 (fT4), and free T3 (fT3)) and assessment of antithyroid antibodies (anti-thyroid peroxidase antibodies and TSHR-Ab). Measurements of serum TSH, FT4, and FT3 were conducted at the laboratory of the Komisarenko Institute using chemiluminescent immunoassay kits (reference TSH range, 0.27-4.2 μIU/mL; refer-ence FT4 range, 0.93-1.71 ng/div; reference FT3 range, 2.02-4.43 pg/mL; reference anti-thyroid peroxidase anti-body range, < 34 Units/mL) on Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Meas-urements of TSH-R-Ab were conducted using chemiluminescent immunoassay kits on a Siemens Immulite 2000 analyzer. Thyroid antibody titers were considered positive for the anti-TSH receptor-Ab titers above 0.55 U/L. The enzyme immunoassays (Vector Best, Novosibirsk, Russia) were employed as per the manufacturer’s instructions to determine the levels of cytokines. Stat Fax 303 Plus microstrip reader (Awareness Technology) was used to determine the bound horse-radish pe-roxidase activity. Sensitivity for IL-1β and IL-10 were 1.0 pg/mL (measurement range, 0-250 pg/mL) and 1.0 pg/mL (measure-ment range, 0-500 pg/mL), respectively, and the reference IL-1β range was 0-11 pg/ml (for 95% of donors). The mean IL-1β value for our 20 female controls was 4.43±0.62. The reference IL-10 range was 0-9 pg/ml, and the mean IL-10 value for the control group (20 women) was 5.32±1.23 pg/ml. Ultrasonography of the thyroid gland was performed using NEMIO SSA-580A system (Toshiba Medical Sys-tems Co, Ltd, Tokyo, Japan) and Ultima RA Gris 941217.01343, with the patient in supine position and the head bent back. Thyroid gland volume was determined by the method of Brunn, with the upper limit of the normal range being 13 mm3. Patients with Graves’ disease were compared with 20 healthy female controls (age, 36 to 42 years; mean age, 40.1±1.80 years) with regard to anti-thyroid peroxidase Ab, TSHR-Ab, IL-1β and IL-10 levels. The Student t test was used for statistical analyses. The level of significance p ≤ 0.05 was assumed. Data are presented as mean ± SEM. Results Forty-three patients with Graves’ disease were divided into two groups based on the presence or absence of EO. Disease duration was 2.2±0.4 years for patients with GD plus EO versus 3.98±0.74 years for patients with GD only (р<0.05). All patients have been treated with antithyroid drugs (mercazolil and thyrozol) for long and were classified as having compensated thyroid disease at the time of the study. All patients with GD plus AO had a CAS score ex-ceeding 3 (the active stage of EO). Table 1 presents clinical characteristics and laboratory data for patients of both groups.
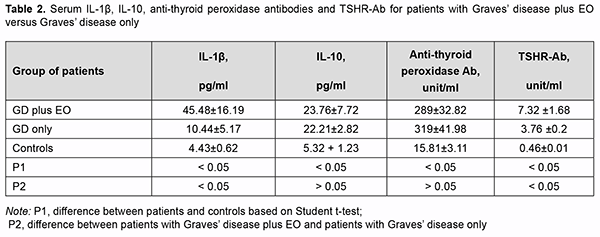
There was no significant difference in TSH, fT4 or fT3 between patients of these groups (Table 1). Thyroid gland dimensions in patients of both groups were significantly higher than normal reference values, with no signifi-cant difference between patients of both groups. Serum IL-1β levels for patients of both groups were significantly higher than for controls (р<0.05). Serum IL-1β levels for patients with GD plus EO were significantly higher than for patients with GD only (45.48 ± 16.19 versus 10.44 ± 5.17; р < 0.05). Table 2 presents serum IL-1β, IL-10, anti-thyroid peroxidase Ab, and TSHR-Ab for patients of both groups. Serum IL-10 levels for patients with GD plus EO and those with GD only (23.76±7.72 and 22.21±2.82, respec-tively) were also significantly higher than for controls (5.32±1.23). There was no significant difference in serum IL-10 or anti-thyroid peroxidase Ab levels between patients of these groups (Table 2).
Serum TSHR-Ab for patients were higher than reference values, and serum TSHR-Ab levels for patients with GD plus EO were significantly higher than for those with GD only (7.32±1.68 unit/mL versus 3.76±0.2 unit/mL; р=0.04). These findings are in agreement with the literature, since serum TSHR-Ab is a diagnostic marker of autoimmune activity in EO. Discussion Therefore, we found significantly increased serum pro-inflammatory cytokine IL-1β and TSHR-Ab levels in pa-tients with GD plus EO compared to those with GD only (45.48±16.19 pg/ml versus 10.44±5.17 pg/ml; р<0.05). This may indicate the specificity of this characteristic as a diagnostic marker of orbital autoimmune activity. Our findings are in agreement with others who reported on the activation of orbital fibroblasts in EO, with these fibroblasts being able to secrete proinflammatory cytokines IL-1β, IL-1α, IL-6, and IL-8 to contribute to orbital inflammation [17]. IL-10 is a cytokine which not only suppresses cellular immunity but also stimulates the humoral response. The current study found significantly increased serum IL-10 levels in patients with GD plus EO and those with GD only, which was as expected accompanied by high serum TSHR-Ab levels. There was, however, no significant difference in serum IL-10 levels between patients with GD plus EO and those with GD only (23.76±7.72 pg/ml versus 22.21±2.82 pg/ml). Therefore, there was no expected response (no increase in serum anti-inflammatory cytokine IL-10 levels with an increase in serum pro-inflammatory IL-1β levels) in patients with GD plus EO. Others have reported similar findings, concluding that mRNA IL-10 expression can be increased in autoimmune thyroid diseases, GD and Hashimoto thyroiditis, and the role of IL-10 might be directed to the stimulation of B cell proliferation and antibody production rather than to the suppression of proinflammatory cytokine release [18]. Conclusion First, serum levels of pro-inflammatory cytokine IL-1β in patients with Graves’ disease were significantly in-creased compared to healthy controls (p < 0.05). Second, serum levels of IL-1β in patients with the active stage of EO in the presence of Graves’ disease were significantly increased compared to patients without EO (45.48 ± 16.19 pg/ml versus 10.44 ± 5.17 pg/ml; p < 0.05), which may indicate the specificity of this cytokine as a marker of in-flammatory autoimmune activity in the orbit. Finally, serum levels of pro-inflammatory cytokine IL-10 in patients with Graves’ disease were significantly increased compared to healthy controls, but there was no significant differ-ence in IL-10 between patients with EO and patients without EO (23.76 ± 7.72 pg/ml versus 22.21 ± 2.82 pg/ml; p > 0.05).
References 1.Gianoukakis AG, Khadavi N, Smith TJ. Thyroid. 2008 Sep;18(9):953-8. doi: 10.1089/thy.2007.0405. 2.Guo H, Callaway JB, Ting JP-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015 Jul;21(7):677-87. doi: 10.1038/nm.3893. 3.Nasonov EL. [The role of interleukin 1 in the development of human diseases]. Nauchno-prakticheskaia revmatologiia. 2018;56:19-27. Russian. 4.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007 Apr 12;356(15):1517-26. doi: 10.1056/NEJMoa065213. 5.Nasonov EL, Eliseev MS. [Interleukin-1 and its role in human pathology]. Terapevticheskii arkhiv. 1987;12:112-7. Russian. 6.Il’ina AE, Stanislav ML, Denisov LN, Nasonov EL. [Interleukin-1 as an inflammatory mediator and therapeutic target]. Nauchno-prakticheskaia revmatologiia. 2011;49(5):62-71. Russian. 7.Dinarello SA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25(6):469-84. 8.Gabay G, Lamacchia C, Palme G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010 Apr;6(4):232-41. doi: 10.1038/nrrheum.2010.4. 9.Lopalco G, Cantarini L, Vitale A, et al. Interleukin-1 as common denominator from autoinflammatory to autoimmune disorders: premises, perils and perspectives. Mediators Inflamm. 2015;2015:194864.. doi: 10.1155/2015/194864 PMCID: PMC4345261. 10.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of new approach. Pharmacological Reviews. June 2003;55(2):241-69. DOI: https://doi.org/10.1124/pr.55.2.4. 11.Liashenko AA, Uvarov VIu. [On the systematization of cytokines]. Uspekhi sovremennoi biologii. 2001;121(6):589-603. Russian. 12.Kharintsev VV, Serebriakova OV, Serkin DM, et al. [Role of some proinflammatory and anti-inflammatory cytokines in the course of endocrine ophthalmopathy]. Zabaikalskii meditsinskii vestnik. 2016;2:33-40. Russian. 13.Wong KH, Rong SS, Chong KK, et al. Genetic associations of interleukin-related genes with Graves' ophthalmopathy: a systematic review and meta-analysis. Sci Rep. 2015 Nov 18;5:16672. doi: 10.1038/srep16672. 14.Slowik M, Urbaniak-Kujda D, Bohdanowicz-Pawlak A, et al. CD8+CD28-lymphocytes in peripheral blood and serum concentra-tions of soluble interleukin 6 receptor are increased in patients with Graves᾿ orbitopathy and correlate with disease activity. En-docr Res. 2012;37(2):89-95. doi: 10.3109/07435800.2011.635622. 15.Fang S, Huang Y, Zhong S, et al. Regulation of orbital fibrosis and adipogenesis by pathogenic Th17 cells in Graves᾿ orbitopathy. J Clin Endocrinol Metab. 2017 Nov 1;102(11):4273-4283. doi: 10.1210/jc.2017-01349. 16.Antonelli A, Ferrari S, Fallahi P, et al. Monokine induced by interferon γ (IFNγ) (CXCL9) and IFNγ inducible T-cell α-chemoattractant (CXCL11) involvement in Graves’ disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-γ agonists. J Clin Endocrinol Metab. 2009 May;94(5):1803-9. doi: 10.1210/jc.2008-2450. 17.Khong JJ, McNab AA, Ebeling PR, et al. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016 Jan;100(1):142-50. doi: 10.1136/bjophthalmol-2015-307399. 18.de la Vega JR, Vilaplana JC, Biro A, et al. IL-10 expression in thyroid glands: protective or harmful role against thyroid autoim-munity? Clin Exp Immunol. 1998 Jul;113(1):126-35. doi: 10.1046/j.1365-2249.1998.00628.x. Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|