J.ophthalmol.(Ukraine).2021;4:32-38.
|
http://doi.org/10.31288/oftalmolzh202143238 Received: 04 March 2021; Published on-line: 16 August 2021 Avoiding errors in the diagnosis of polypoidal choroidal vasculopathy in patients with age-related macular degeneration (Part 1) Lutsenko N. S., Rudycheva O. A., Isakova O. A., Kyrylova T. S. State institution "Zaporizhzhia Medical Academy of Postgraduate Education of the Ministry of Health of Ukraine"; Zaporizhzhia (Ukraine) E-mail: rudychevaolga5@gmail.com TO CITE THIS ARTICLE: Lutsenko NS, Rudycheva OA, Isakova OA, Kyrylova TS. Avoiding errors in the diagnosis of polypoidal choroidal vasculopathy in patients with age-related macular degeneration (Part 1). J.ophthalmol.(Ukraine). 2021;4:32-38. http://doi.org/10.31288/oftalmolzh202143238 Background: Poor availability of indocyanine green angiography (ICGA) raises the question of how to improve the diagnosis of polypoidal choroidal vasculopathy (PCV) using alternative imaging modalities, optical coherence tomography (OCT) and OCR angiography (OCTA). Purpose: To optimize the diagnosis of PCV in patients with age-related macular degeneration (AMD) using non-invasive methods, OCT and OCTA. Material and Methods: One hundred and sixty-nine patients (228 eyes) with neovascular AMD underwent OCT and OCTA study as per the proposed algorithm to differentiate between PCV and other types of subretinal neovascularization. Of these, 14 patients (8.28%; 14 eyes) were found to have PCV. Results: Major and additional signs of PCV, secondary neovascularization and activity of the process on OCT were determined. In addition, we determined the signs of PCV on OCTA manu-al and automatic segmentation which will be helpful in accurate differential diagnosis of the disease. On the basis of these data, we developed a step-by-step algorithm of the OCT and OC-TA diagnosis of PCV in patients suspected of the disease, which allows the accurate diagnosis when ICGA is unavailable. Conclusion: Systematic step-by-step interpretation of OCT and OCTA scans allow the reliable differential diagnosis of PCV in patients with exudative AMD. Keywords: optical coherence tomography, optical coherence tomography angiography, age-related macular degeneration, polypoidal choroidal vasculopathy, differential diagnosis
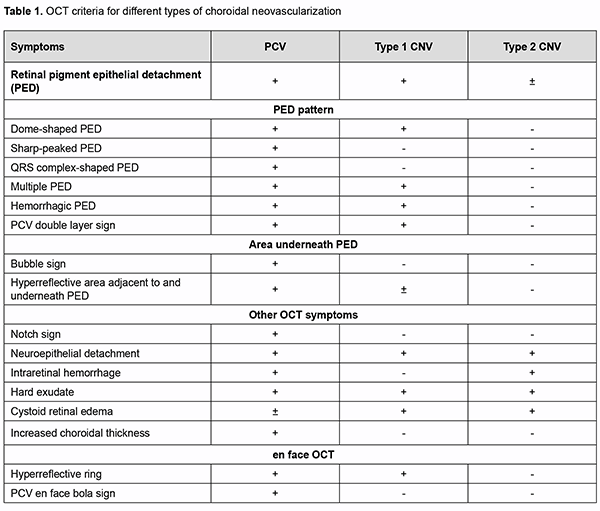
Introduction Advances in diagnostic technologies for eye diseases and advent of optical coherence tomography (OCT) and OCR angiography (OCTA) have substantially improved the diagnosis of age-related macular degeneration (AMD) [1, 2, 3, 4, 5, 6]. Pathological morphological and structural changes and/or microcirculatory changes in the retina can be easily identified by current diagnostic methods. Identifying the type and features of subretinal neovascular membranes (SNM) is, however, important in making the diagnosis and guiding treatment decisions. Considering these aspects is especially important when making the diagnosis of rare types of neovascularization such as poly-poidal choroidal vasculopathy (PCV) [6, 7, 8, 9, 10, 11, 12]. Although some experts believe that PCV is a subtype of neovascular AMD, others believe that it is a separate clinical entity [13, 14, 15, 16, 17, 18]. Unlike other types of AMD, PCV is the only type of neovascularization which can be seen commonly in in-creased choroid thickness and less commonly in normal or reduced choroid thickness [7, 8, 10, 17]. OCT and indocyanine green angiography (ICGA) are common imaging techniques for the diagnosis of PCV [7, 10, 11, 14, 17, 18]. The diagnostic algorithm developed by the Japanese Study Group of Polypoidal Choroidal Vasculopathy (2005) was based on the clinical data and ICGA data; it has significantly improved the diagnosis of PCV, and was the major generally accepted diagnostic approach until the advent of OCTA, a new method for the diagnosis of fundus pathology [4, 7, 11]. The latter method is non-invasive and allows detailed 3-D layer-by-layer evaluation of the retinal and choroidal vascular network. The diagnostic value of OCTA in suspected PCV has been demonstrat-ed in numerous studies [2, 4, 7]. Some authors have proposed advanced PCV diagnostic algorithms on the basis of clinical and instrumental findings, but not on ICGA data [4, 15]. Although the ICGA has straightforward ad-vantages for the diagnosis of PCV, it also has disadvantages which should be taken into consideration. First, the application of indocyanine green dye may lead to general complications as mild as nausea to as severe as anaphy-lactic shock. Second, there are contraindications to having the ICGA (immune changes, hepatic failure, renal fail-ure, etc.). Finally, indocyanine green dye and facilities for performing the ICGA are not always readily available. Therefore, we believe that developing algorithms for the non-invasive diagnosis of PCV is important. The purpose of the study was to optimize the diagnosis of PCV in patients with AMD through the use of non-invasive methods, OCT and OCTA. Material and Methods One hundred and sixty-nine patients (228 eyes) with neovascular AMD were examined. Of these, 14 patients (8.28%; five men and nine women; 14 eyes) were found to have PCV. Mean patient age was 65 ± 7.7 years. Patients underwent an eye examination, including visual acuity, perimetry and tonometry. In addition, they un-derwent a multimodal fundus examination including ophthalmoscopy, scanning laser ophthalmoscopy, OCT, en face OCT and OCTA. Visual acuity ranged from 0.3 to 0.7. This prospective and non-controlled study was performed at the Zaporizhzhia Regional Clinical Hospital from January 2020 to September 2020. Patients with neovascular AMD were included. The exclusion criterion was my-opic, posttrombotic, diabetic, or post-inflammatory neovascularization not associated with AMD. This study was performed within the framework of a planned research design for the department project (VN.R.01.05-19 №0019U101932). The study protocol was approved by the Bioethics Committee (Committee Minutes dated 14.01.2019). The conduct of the study adhered to the Declaration of Helsinki. Informed consent was obtained from all participants. The AngioVue system (RTVue XR OCT Avanti, Optovue, Inc., Fremont, CA) was used for split-spectrum ampli-tude-decorrelation angiography (SSADA) measurements with OCT and OCTA. Retinal OCT was performed using Cross Line, Retina Map, and 3D Widefield scans. We used OCT to measure choroidal thickness manually at the foveal center and determine maximum thickness. The AngioRetina 3 × 3 mm and 6 × 6 mm scan protocols were used to perform OCTA in the macular area. Angiography was assessed using manual and automatic segmentation modes. Results and Discussion Given that OCT is a major diagnostic technique for the detection of macular pathology which has demonstrated sensitivity and specificity in the diagnosis of PCV as high as 87.5 – 94.6% and 86.2 – 92.9%, respectively, we be-lieved that it was reasonable to begin a step-by-step process of the diagnosis of PCV from the analysis of retinal morphological changes without taking clinical manifestations into consideration [19, 20, 21, 22, 23, 24]. This diag-nostic approach on the basis of objective data only helps a clinician screen for fundus pathology and suspect PCV. Thus, on the one hand, the absence of complaints and ophthalmoscopic fundus changes and the presence of high visual acuity in these patients cannot exclude the presence of “quiescent” polyp in the absence of any intraretinal or subretinal fluid or hemorrhage in the early disease, which can give a false opinion and hampers early diagnosis of PCV. On the other hand, in the presence of neovascular complex activity, the ophthalmoscopic signs are marked but ambiguous. Thus, a major clinical feature of PCV, orange–red subretinal nodules, may not be observed for a number of reasons (extensive hemorrhagic changes, etc.). In addition, hard exudate is characteristic of PCV but may be seen in some other types of neovascular AMD as well, whereas drusen are not typical of PCV, but may be seen in the fellow eye of the unilateral PCV patient. Such variability of clinical manifestation in the presence of an active neovascular complex may make the dif-ferential diagnosis difficult at initial diagnosis of PCV. The above explains why our approach to the diagnosis of PCV was based on the detection of retinal morphological changes only. Therefore, at the first phase of this study, we determined distinctive OCT features that are characteristic of neo-vascular AMD on the basis of literature review [1, 4, 6, 7, 9, 10, 13, 14, 17,18, 22]. The findings of this review are presented in table 1.
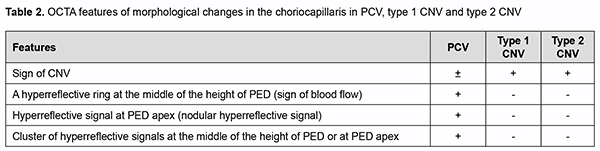
The morphological changes that are characteristic of PCV only have been noted. Although retinal pigment epi-thelial detachment (PED) is present in any type of neovascularization and evidences the presence of neovasculari-zation, the clinician should determine whether additional OCT scan features characteristic of PCV (thumb like, sharp-peak and QRS complex shaped PEDs) are present or not. Therefore, in order to detect PCV, it is important to take into consideration the type of PED, whereas other OCT features seen in different types of neovascularization are not important for the diagnosis of PCV and just give evidence of the activity of the process and presence of exudative only or exudative and hemorrhagic changes. Another important aspect of the diagnosis is to determine whether the OCT features directly confirming the presence of pathological neovascular network in the retinal and choroidal structures can be seen. A Double-layer Sign (DLS) is present in some types of neovascular AMD and is pathognomonic of pathologic neovascular network in the retina or choroid. Because the cause of PCV is considered to be due to inner choroidal vessel abnormalities, the pathological vascular network in eyes with PCV is different from that in eyes with SNM. Notch sign and bubble sign are additional OCT features which are present in PCV only. OCT assessment of the choroidal thickness at the fovea helps a clinician differentiate between various forms of neovascular AMD. There have been numerous studies on this subject. Margolis and Spaide [19] measured cho-roidal thickness in normal eyes at different points using enhanced depth imaging (EDI) OCT and to evaluate the association of choroidal thickness and age. The mean age was 50.4 years, and the choroid was thickest underneath the fovea (mean, 287 microm). Regression analysis suggested that the subfoveal choroidal thickness decreased by 15.6 microm for each decade of life. Some studies measured choroidal thickness in eyes with PCV and found that the choroid was thicker in these eyes than in normal eyes. Choroidal thickness, however, tends to be normal or de-creased in other subtypes of neovascular AMD. Therefore, at initial diagnosis of PCV (when making an initial diag-nosis), OCT scan data should be considered in the following sequence: one or more retinal PED characteristic of PCV (dome-shaped, thumb like, sharp-peaked and QRS complex shaped PEDs); additional OCT features which are present in PCV only (notch sign and bubble sign); a Double-layer Sign (DLS) confirming the presence of SNM; de-termining whether the process is active (hyperreflective hemorrhage, hard exudate, neuroepithelial detachment, and cystic changes in the macula), and assessing choroidal thickness by OCT in order to exclude other subtypes of neo-vascular AMD. Errors at initial diagnosis may occur due to (1) areas of apparent exudate associated with the well-developed neovascularization or (2) low height of the polyp which makes the OCT features of PCV less obvious, suggesting the presence of SNM or other subtypes of neovascular AMD. Therefore, the disease may remain unrecognized. In the current study, comprehensive evaluation (at the initial diagnosis) revealed a diagnosis of suspected PCV in 68 eyes, with 28 patients having a diagnosis of unilateral suspected PCV. Choroidal thickness at the fovea ranged from 182 to 436 μm, with a mean of 281.2 ± 42.54 μm. The choroid was thicker than normal (with evidence of dilated Haller's layer vessels) in 6 eyes, of normal thickness in 9 eyes, and thinner than normal in 53 eyes. The polyp detection (i.e., detection of anatomical and functional changes in the retinal and choroidal microvas-cular bed) is pathognomonic of the final diagnosis of PCV. Until recently, ICGA has been the only method for the diagnosis of the disease. However, the poor availability of ICGA in Ukraine, evidence of the advantages of OCTA in the diagnosis of the pathology and our extensive personal experience accumulated with OCTA provided the ra-tionale for employing OCTA for making the final diagnosis of PCV. We took in consideration the literature review to define typical OCTA features of PCV, and, on the basis of these features, one can differentiate between the types of neovascularization [4, 7, 9, 10, 16, 17, 18] (Table 2).
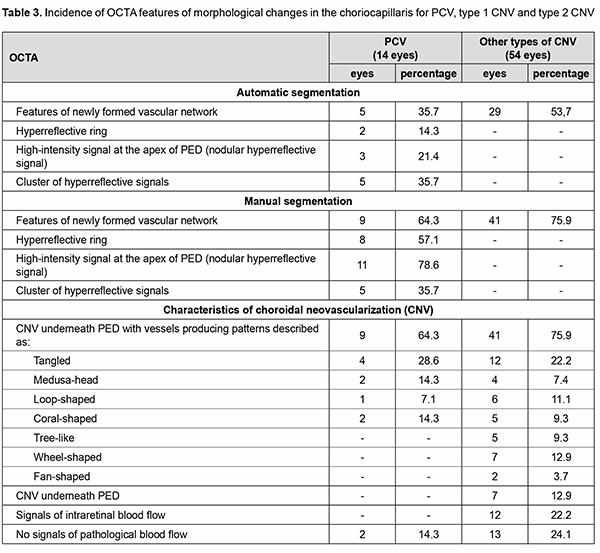
Analysis of the results obtained showed that typical OCTA features of PCV do exist on the basis of which one can differentiate between the types of neovascularization, thus making the next and final step in the diagnosis of PCV. While assessing the results obtained with automatic segmentation of OCTA, it is important to determine cor-rectly the location of pathological blood flow signal (“ring” sign, nodular hyperreflective signal or point-shaped sig-nal at polyp area, and pathological vascular network), particularly, in the choroid, outer retina, and deep vascular plexus, which is characteristic of PCV. One should take into account the fact that due to apparent changes associ-ated with exudation and hemorrhage, segmentation may be altered, with abnormal blood flow signals localized to the wrong layers [25]. This will lead to substantial errors and final misdiagnosis. We believe that these errors may be avoided with manual segmentation of OCTA. Using the method only for detecting and assessing choroidal neovas-cularization, however, is not enough for making the differential diagnosis of PCV and determining the type of pathological vascularization. The detection of blood flow signal at the area of supposed polyp is nevertheless a key point in making the final diagnosis of PCV. In addition, selection of manual segmentation settings and scan direc-tion settings are important, and polypoidal complexes should be assessed beginning from the choroid and ending at PED apex with the use of en face slices not thicker than 30 µm, because ring-shaped blood flow signals can be de-tected at PED midheight or higher, whereas nodular hyperreflective signals, at PED apex. These symptoms are spe-cific of PCV, and the detection of blood flow signals in these zones will confirm the presence of a polyp or polyps [7, 8, 9, 10, 15]. At this phase of the diagnostic process, it is important also to take into consideration quantitative assessment of polyps, a major component of PCV. One should remember that there may be either one or multiple polyps, and multiple polyps may adhere to each other in clusters, which is not characteristic of other types of neo-vascularization. Given the above, we used our algorithm for assessing OCTA findings in 169 patients (228 eyes) with neovascu-lar AMD, and, of these, 14 eyes were found to have PCV. The algorithm for analysis of OCTA findings was as follows. Step 1. Selection of manual segmentation settings and scan direction settings Detecting neovascularization in the area adjacent to or underneath PED. In PCV, neovascularization is ob-served only in the area adjacent to dome-shaped PED. Use en face slices not thicker than 30 µm and review B scans for the detection and confirmation of blood flow signals. In order to assess polypoidal complexes (areas of the PEDs characteristic of PCV), shift the en face slice step by step from the choroid towards PED apex. When the slice is shifted upward from the choroid, the clinician detects neovascularization and then other blood flow signals. Step 2. Polyp number assessment There may be either one or multiple polyps, and multiple polyps may adhere to each other in clusters. Step 3. Polyp blood flow evaluation Ring-shaped blood flow signals can be detected at PED midheight or higher, whereas nodular hyperreflective signals, at PED apex. This feature is specific of PCV, and the detection of blood flow signals in the regions of sus-pected polyps will confirm the presence of PCV. Step 4. Assessment of choroidal neovascularization characteristics Choroidal neovascularization that is adjacent to the vascular complex underneath PED corresponds to the loca-tion of double layer sign on the OCT. The branching vascular network may exhibit patterns described as “tangled”, “loop-shaped”, “medusa-head”, etc. Step 5. If there is no abnormal blood flow signal All cases of suspected pathological neovascularization without abnormal blood flow signal should be identified if there is evidence of hemorrhagic PED and neuroepithelial detachment or PED with marked exudation. In these cases, other research methods are not helpful in confirming the presence of vascular changes. Only clinical analysis and longitudinal condition monitoring in the course of treatment will be helpful in making the correct diagnosis. Table 3 presents the results of assessment of OCTA signs in patients with PCV and SNM of other types.
Therefore, the OCT-based analysis of retinal morphological changes with consideration of specific symptoms allowed us to identify a group of patients with suspected PED (68 eyes; 30%). Subsequently, an OCTA study of patients with consideration of the proposed diagnostic algorithm allowed us to confirm the diagnosis of PED in 14 of 169 patients (8.28%). The developed approach to OCTA study in patients with suspected PED allows the clini-cian to (1) avoid errors in determining the type of neovascularization and (2) make a correct diagnosis when ICGA is unavailable. Conclusion The detection of blood flow in suspected polyp by angiographic methods visualizing choroidal blood flow (ICGA, a gold-standard for diagnosing PCV, and OCTA) is a reliable criterion for the diagnosis of the disease. Man-ual segmentation and systematic step-by-step interpretation of OCTA scans allows the clinician to detect specific symptoms and signs of PCV and generate an adequate differential diagnosis when ICGA is unavailable.
References 1.Ducker DS, Weheed ND, Goldman DR. Optical coherence tomography of the retina. 2nd ed. Moscow: MEDpress-inform; 2019. 2.Lutsenko NS, Rudycheva OA, Isakova OA, Kyrylova TS. Assessing OCTA changes in morphology and structure of retinal micro-vascular bed in patients with exudative AMD. J Ophthalmol. 2019;2:7-13. 3.Lutsenko NS, Isakova OA, Rudycheva OA, Kyrylova TS, Unguryan NV. [Macula. Today's diagnostics. (Optical coherent tomog-raphy and Optical coherent tomography angiography)]. Zaporizhzhia: Orbita-Yug Agency; 2019. Ukrainian 4.Shaimov TB, Panova IP, Shaimov RB, Shaimov TA, Fomin AV. [Optical coherence tomography-angiography in the diagnosis of neovascular age-related macular degeneration]. Bull of Ophthalmology. 2015 Sep-Oct;131(5):4-13. Russian. 5.Lumbroso B, Huang D, Jia Y, Rispoli M, Fujimoto JG. Clinical OCT Angiography Atlas. 1st ed. New Delhi: Jaypee Brothers medical Publishers; 2015. 6.Miotto S, Zemella N, Gusson E, Panozzo G, Saviano S, Scarpa G, Boschi G, Piermarocchi S. Morphologic criteria of lesion activi-ty in neovascular age-related macular degeneration: a consensus article. J Ocul Pharmacol Ther. 2018 Apr;34(3):298–308. 7.Seong S, Choo HG, Kim YJ, Kim JY, Lee JH, Oh HS, Yuo YS, Kim SH, Kwon OW. Novel Findings of Polypoida Choroidal Vasculopathy via Optical Coherence Tomography Angiography. Korean J Ophthalmol. 2019 Feb;33(1):54-62. 8.Panova IE, Shaimov TB, Shaimova VA. [Non-Invasive Diagnosis of Polypoidal Choroidal Vasculopathy as a Variant of the Course of Age-Related Macular Degeneration]. Ophthalmology in Russia. 2018;15(2S):273-280. Russian 9.Shaimov TB, Panova YE, Shaimova VA. [Algorithm for non-invasive clinical and instrumental diagnostics of polypoid choroidal vasculopathy. Modern technologies in ophthalmology]. Russ ophthalmol onl [Internet]. 2018 Jun 14;1(21):402-7. Russian 10.Shaimov TB, Panova YE, Shaimova VA. [Differential diagnostic criteria in the diagnosis of polypoid choroidal vasculopathy as a variant of the course of the neovascular stage of age-related macular degeneration. Modern technologies in ophthalmology]. Russ ophthalmol onl [Internet]. 2017 Mar 1;1(14):335–9. Russian 11.Yang J, Yuan M, Wang E, Xia S, Chen Y. Noninvasive multimodal imaging in diagnosing polypoidal choroidal vasculopathy. BMC Ophthalmol. 2019 Nov 16;19(1):229-236. 12.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1–8. 13.Mayorova AM. [Polypoid choroidal vasculopathy as a subtype of age-related macular degeneration: diagnosis and treatment]. Dis-sertation for the degree of Dr Sc (Med). Moscow. 2018. Russian. 14.Wong CW, Wong TY, Cheung CMG. Polypoidal Choroidal Vasculopathy in Asians. J Clin Med. 2015 Apr 24;4(5):782-821. 15.Wang M, Zhou Y, Gao SS, Liu W, Huang Y, Huang D, Jai Y. Evaluating polypoidal choroidal vasculopathy with optical coher-ence tomography angiography. Invest Ophthalmol Vis Sci. 2016 July;57(9):526-532. 16.Tomiyasu T, Nozaki M, Yoshida M, Ogura Y. Characteristics of polypoidal choroidal vasculopathy evaluated by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016 July;57(9):324-30. 17.Anantharaman G, Sheth J, Bhende M, Narayanan R, Natarajan S, Rajendran A, Manayath G, Sen P, Biswas R, Banker A, Gupta C. Polypoidal choroidal vasculopathy: Pearls in diagnosis and management. Indian J Ophthalmol. 2018 Jul;66(7):896–908. 18.Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen S, Chen Y, Freund KB, et al. Polypoidal Choroidal Vasculopathy. Definition, Pathogenesis, Diagnosis, and Management. Ophthalmolology. 2018 May;125(5):708-724. 19.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009 May;147(5):811-5. 20.De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G. Sensitivity and specificity of spectral-domain optical coherence tomogra-phy in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014 Dec;158(6):1228-38. 21.Kim JH, Kang SW, Kim TH, Kim SJ, Ahn J. Structure of polypoidal choroidal vasculopathy studied by colocalization between tomographic and angiographic lesio. Am J Ophthalmol. 2013 Nov;156(5):974-980. 22.Lui R, Li J, Yu S, Yang Y, Yan H, Zeng J, Tang S, Ding X. Distinguishing polypoidal choroidal vasculopathy from typical neo-vascular agerelated macular degeneration based on spectral domain optical coherence tomography. Retina. (Philadelphia, Pa). 2016 Apr;36(4):778-786. 23.Sato T, Kishi S, Watanabe G, Matsumoto H, Mukai R. Tomographic features of branching vascular networks in polypoidal cho-roidal vasculopathy. Retina. 2007 Jun;27(5):589-94. 24.Tsujikawa A, Sasahara M, Otani A, Gotoh N, Kameda T, Iwama D, Yodoi Y, Tamura H, Mandai M, Yoshimura N. Pigment epi-thelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007 Jan;143(1):102-111. 25.Lauermann JL, Woetzel AK, Treder M, Alnawaiseh M, Clemens CR, Eter N, Alten F. Prevalences of segmentation errors and mo-tion artifacts in OCT-angiography differ among retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2018 Oct;256(10):1807-1816. Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|