J.ophthalmol.(Ukraine).2021;4:26-31.
|
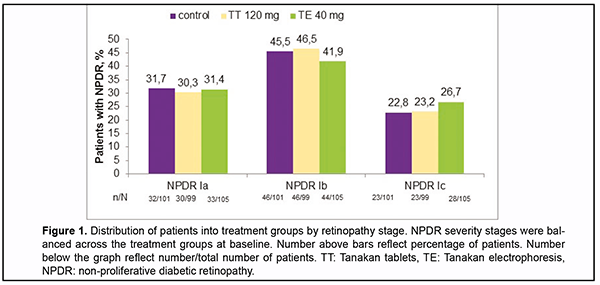
http://doi.org/10.31288/oftalmolzh202142631 Published on-line: 16 August 2021 Prediction of the progression of diabetic retinopathy based on hemodynamic data F. A. Bakhritdinova 1, MD, professor; G. E. Kangilbaeva 1, PhD, assistent; I. F. Nabieva 2, doctor; A. Z. Jurabekova 3 , doctor 1 Tashkent Medical Academy; Tashkent (Uzbekistan) 2 Clinic of Research Institute of Endocrinology; Tashkent (Uzbekistan) 3 Nazar Medical Eye Clinic; Tashkent (Uzbekistan) E-mail: doctorguzal70@gmail.com TO CITE THIS ARTICLE: Bakhritdinova FA, Kangilbaeva GE, Nabieva IF, Jurabekova A.Z. Prediction of the progression of diabetic retinopathy based on hemodynamic data. J.ophthalmol.(Ukraine). 2021;4:26-31. http://doi.org/10.31288/oftalmolzh202142631 Background. Diabetic retinopathy (DR) is a vascular complication of diabetes, leading to vision loss and blindness in people of any age. This highlights the need for monitoring and predicting the course of DR to start early timely treatment and to prevent progression to the proliferative stages of the disease. Purpose. To study the features of ocular hemodynamics and develop the progression risk index for DR. Material and Methods. One hundred and sixty-five patients with non-proliferative diabetic retinopathy (NPDR) were randomly allocated to receive traditional treatment (control group), daily tablets of Tanakan (TT group), or daily endonasal electrophoresis of Tanakan (TE group) within ten days. The stages of DR were determined using the severity scale of the Early Treatment Diabetic Retinopathy Study (ETDRS). The main outcome measures were changes in DR severity (DRS), Doppler ultrasound imaging at months 1, 3, and 6. Results. Moderate negative correlations were found between peak systolic velocity (PSV) of the central retinal artery (CRA) and DRS, end-diastolic velocity (EDV) of CRA and DRS, PSV of central retinal vein (CRV) and DRS, PSV of the short posterior ciliary artery (SPCA) and DRS. Developed diabetic retinopathy progression risk index (DRPRI) was less than 1,0 in the TE group that enabled us to predict DR stability or improvement. Conclusions. This validation study demonstrated that PSV and DRS are correlated, and PSV can be used to diagnose the stages of DR. Developed DRPRI can be used to assess the effectiveness of treatment and to predict DR, which is essential for determining further patient management tactics. Кey words: disease prognosis, diabetic retinopathy, peak systolic velocity, resistivity index, diabetic retinopathy severity, progression risk index. Introduction The growing trend in the number of patients with diabetes mellitus (DM), reached 463 million people worldwide in 2019 [1], making DM an essential public health issue, with considerable socioeconomic and quality-of-life con-sequences. Diabetic retinopathy (DR) is a vascular complication of diabetes, leading to vision loss and blindness in people of any age. The pathophysiology of DR are diverse and not fully understood. However, most authors identi-fy a wide range of metabolic disorders caused by hyperglycemia, vascular endothelial disorders, pericyte deficiency, and vascular hyperpermeability [2, 3]. In diabetes mellitus, сhanges in the blood rheological properties lead to capillary occlusion and impaired vessel integrity [4, 5]. Retinal non-perfusion areas induce the overproduction of mitochondrial superoxide and pro-angiogenic factors [6]. Aznabaev et al.[7,8] showed that the blood flow velocity in the retinal and orbital arteries increases in patients with mild non-proliferative diabetic retinopathy (NPDR). Hyperglycemia probably increases blood flow, which is a compensating mechanism to provide blood supply and maintain high levels of metabolism in the eye tissue. In other studies, the authors found that the blood flow velocity in the ocular vessels was already de-creased at the mild stage of NPDR [9, 10]. Various studies of ocular blood flow in DR are contradictory. It is, there-fore, necessary to continue the investigation of ocular hemodynamics and its correlation with the retinopathy sever-ity. Currently, panretinal photocoagulation (PRP) and anti-vascular endothelial growth factor (VEGF) therapy are the most effective way to prevent blindness in the severe non-proliferative and proliferative stages of DR [11, 12, 13, 14]. PRP is used to ablate ischemic neurons and glial cells in non-perfusion areas, thereby reducing the produc-tion of pro-angiogenic factors. PRP also obliterates new vessels with increased permeability, avoiding retinal and vitreous hemorrhages, and creates chorioretinal adhesions that prevent tractional retinal detachment [15, 16, 17]. However, despite the PRP, proliferative retinopathy may be complicated by vitreous hemorrhage or tractional reti-nal detachment. Then vitreoretinal surgery is performed as the only possible and necessary interventions to prevent irreversible loss of vision. This highlights the need for monitoring and predicting the course of DR to start early time-ly treatment and to prevent progression to the proliferative stages of the disease. The objective of this post hoc analyses was to explore the features of ocular hemodynamics, determine the criteria for diabetic retinopathy pro-gression, and develop the progression risk index for diabetic retinopathy. Material and Methods The study included 165 patients (305 eyes) with both types of DM who had non-proliferative diabetic retinopa-thy (NPDR). Age ranged from 18 to 79 years; women were 94, men 71. The exclusion criteria were eye diseases such as glaucoma, inflammatory eye diseases, pigmented and other retinal dystrophies, retinal detachments, eye injury or intraocular surgery with the prior six months, as well as severe somatic diseases such as kidney or liver fail-ure, glucose-galactose malabsorption (GGM), hypertensive crisis. All patients signed informed consent for treatment and examination. Patients with NPDR were randomly allocated to receive traditional treatment (hypoglycemic treatment, noot-ropil, statins, fenofibrats, angioprotectors) as the control group, in addition to the traditional treatment daily tablets of EGb 761 (Tanakan, Beaufour Ipsen Industrie, Paris, France) 120 mg as the TT group, or daily endonasal elec-trophoresis of Tanakan 40 mg (permission of the National Ethics Committee of the Ministry of Health of the Re-public of Uzbekistan No. 11 of 2.11.2010) as the TE group, within ten days. All of the patients underwent baseline ophthalmologic examinations, including visual acuity measurement using a Snellen chart, retinal photosensitivity, intraocular pressure measurement, anterior segment slit-lamp, and fundus examinations using 60, 90D (Volk) lenses, Goldman three-mirror lens, and Mainster Standart fundus lens. Besides, we studied the antioxidant activity of tears, Doppler ultrasound imaging of the eye using an ultrasound system for general clinical HD 11XE (Philips), and HI VISION Preirus (Hitachi). Patients were also examined at day 10 and at months 1, 3, 6. The most informative and statistically significant criteria for the diabetic retinopathy progression were the findings of fundus examinations and Doppler ultrasound imaging. Therefore, in this article, we present the results of these research methods. The stages of DR were determined using the severity scale of the Early Treatment Diabetic Retinopathy Study (ETDRS), the "gold" standard for a detailed assessment of the fundus condition in sci-entific studies worldwide. All statistical analyses were performed using the Statistical Package for the Social Scienc-es (SPSS) software version 23. P values < 0.05 were considered statistically significant. Results Patients included in the study had varying levels of NPDR severity: mild Ia (ESDRS, 14-40 weighted kappa), moderate Ib (ESDRS, 41-60), and severe Ic (ESDRS, 61-80). The number and size of hard exudates, cotton wool spots, retinal hemorrhages, microaneurysms, venous anomalies, and macular edema were evaluated. At baseline, NPDR severity levels were balanced across the treatment groups (Fig.1).
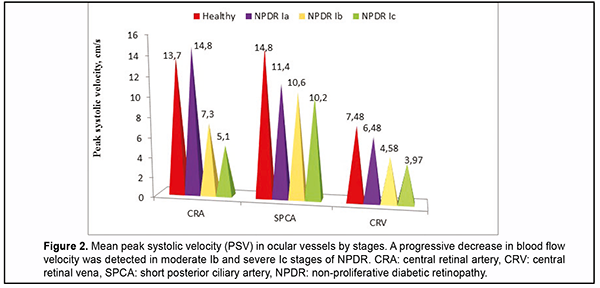
Increased blood flow in the initial Ia stage of NPDR and a progressive decrease in the second Ib and third Ic stage of NPDR in the peak systolic velocity (PSV) of the central retinal artery (CRA), was detected at baseline. A progressive decrease in blood flow by steps was also detected in the short posterior ciliary artery (SPCA). The peak systolic velocity in the central retinal vein (CRV) changed from 6.5 cm/s in the Ia stage to 3.5 cm/s in the Ic stage, which indicates dilatation of the vein, increasing as retinopathy progresses [18] (Fig.2).
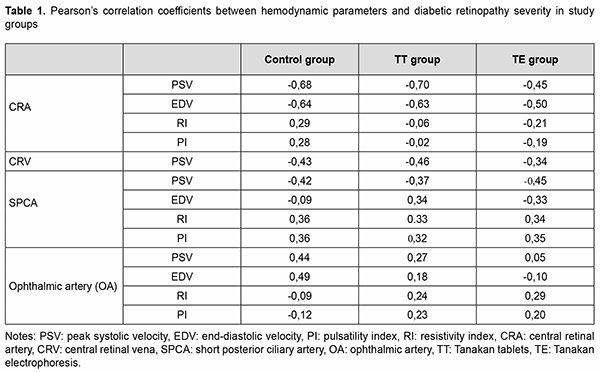
In the correlation analysis (Table 1), a moderate negative correlation was found in all groups between PSV of CRA and diabetic retinopathy severity (DRS) (r = -0.68;-0.70;-0.45), end-diastolic velocity (EDV) of CRA and DRS (r = -0.64; -0.63;-0.50), PSV of CRV and DRS (r =-0.43;-0.46;-0.34), PSV of SPCA and DRS (r = -0.42;-0.37;-0.45). A moderate positive correlation was found between the resistivity and pulsatility indices (RI, PI) of the SPCA and DRS (r = 0.32-0.36). Therefore, such hemodynamic parameters as PSV and EDV of CRA, PSV of CRV, PSV, RI and PI of SPCA can be criteria for the diagnosis of NPDR stages.
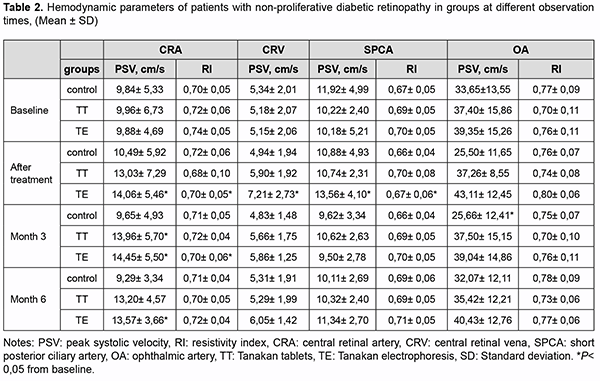
Mean DRS changed from 43.5 to 33 by 24.1% in the TE group, from 41.5 to 34.5 by 16.9% in the TT group, and from 42.5 to 39 by 8.2% in the control group from baseline to day 10 (Fig.3). Moreover, in the control group, we found an increase in SDR by 23.6% at month 6, which indicates the progression of the disease [5]. For Doppler ultrasound imaging, we have found that PSV of CRA increase was not statistically significant (P>0.05) from 9.84±1.11 cm/s at baseline to 10.49±1.24 cm/s by 6.6 % in the control group at day 10 (Table 2). However, patients of this group experienced PSV worsening subsequently. At month 3, hemodynamic parameters of these patients almost returned to the baseline level and worsened by 5.6% from baseline at month 6. In the TT group, the mean PSV of the CRA not significantly increased from baseline 9.96±1.37 to 13.03±1.49 (30.8%) after treatment, and this improvement continued until three months, have reached a significant level (13.96±1.16) from baseline (P<0.05). Likewise, the mean PSV in SPCA also not statistically increased after treatment and remained so throughout the observation period. The complex therapy in the TE group was the most effective. The PSV in the CRA significantly increased (P<0.05) after treatment by 42.3% from baseline, and maintaining significantly high values throughout the observation period. Likewise, RI significantly (P<0.05) decreased after therapy and retained a significant difference from baseline over the observation period (Table 2), which indicates a persistent decrease in peripheral resistance in the pool of the located artery. The same significant improvement in blood velocity after treatment in the TE group was recorded in CRV and SPCA. Likewise, the RI of SPCA significantly decreased, which indicated an improvement in blood rheology and vascular dilatation.
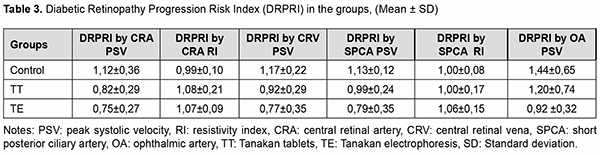
As described previously, the moderate negative correlation between the blood flow velocities and the DRS (Ta-ble 1) is a reason for using CRA, CRV, and SPCA flow velocities to diagnose the stages of DR. Given this correla-tion, we developed and calculated a diabetic retinopathy progression risk index (DRPRI) as the ratio of blood flow velocity before and after treatment: DRPRI = PSV baseline / PSV after 10 days and for the resistive index DRPRI = RI after 10 days / RI baseline (Table 3). Calculated DRPRI below one predicts improvement or stability of diabetic retinopathy. Conversely, the index above 1.0 predicts worsening and progression of diabetic retinopathy.
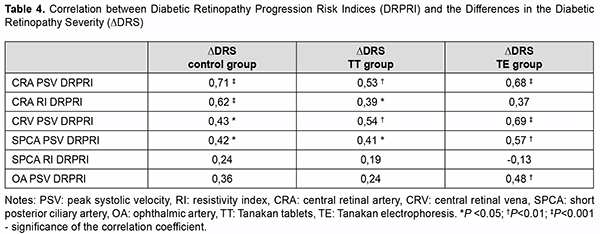
Table 3 demonstrates that the DRPRI calculated using PSV and RI in CRA, CRV, SPCA, and ophthalmic artery (OA) were less than 1.0 in the TE group. This DRPRI enabled us to predict DR stability or improvement. The DRPRI calculated using PSV in CRA and CRV in TT group were slightly more than the DRPRI in TE group, but below 1.0, which gives reason to predict a stable DR. The DRPRI calculated using PSV in the OA was 1.1, and calculated using PSV and RI in SPCA was 1.0, which were the reason for predicting minimal DR progression in TT group. Some con-trol group patients had progression to the proliferative stage of DR at month 6. This progression corresponds to the DRPRI greater than one in control group patients. Patients were observed for six months to validate the developed DRPRI. The difference in the diabetic retinopa-thy severity after 6 months of observation and baseline was calculated: ∆DRS = DRS6month - DRS baseline. The correlation between the ∆DRS and the DRPRI was then studied (Table 4).
In all three groups of patients, significant positive correlations were found between the PSV DRPRI and ΔDRS. This indicated that the larger the calculated PSV DRPRI, the greater the DRS will be in six months, and thus the more signs of DR progression in the long term follow up. Peak systolic velocities in the CRA, CRV, and SPCA were found to be the most reliable for the diabetic retinopathy progression prognosis with correlation coefficients of 0.41-0.71 (Table 4). Discussion These data analyses from Doppler ultrasound imaging demonstrate an increase in eye blood flow rate at the ini-tial Ia stage of DR. Decreased blood flow rate was detected at Ib and Ic stages (Fig.2). Similar data were obtained by Aznabaev еt al. in studies of eye hemodynamics and neuroprotection of non-proliferative diabetic retinopathy [7]. The first signs of DR (microaneurysms, vein dilation) appear in increased blood flow at the initial stage of DR. Blood rheology is then disturbed during prolonged hyperglycemia, adhesion and infiltration of leukocytes damage vascular endothelial cells. Capillary occlusion and subsequently non-perfused ischemic areas appear [2,3,4,17]. The present study demonstrated that endonasal tanakan electrophoresis therapy improves hemoreology, ocular blood flow rate, and DRS. This proves the need for early treatment of DR to achieve the most effectively reduce the risk of progression to the proliferative stage. Several studies have demonstrated the pharmacological effects of tablets of EGb 761 in DR therapy [4,5,6]. Our research showed that Tanakan endonasal electrophoresis treatment was the most effective therapy for improving DRS, maintaining the effect for six months, and reducing the frequency of treatment. Patient monitoring for 6 months confirmed the validity of the developed DRPRI. Several studies have developed and evaluated a prognostic prediction model/tool, using multiple prognostic factors to predict the risk of progression of diabetic retinopathy and a first occurrence of macro- and microvascular complications [19.20,21]. Unlike the previous study, we have developed a progression risk index of diabetic retinopathy using blood flow ve-locity. We confirmed the validity of DRPRI by observing patients for 6 months. Conclusions This validation study demonstrated that PSV and DRS are correlated, and PSV can be used to diagnose the stages of DR. Developed DRPRI below 1.0 indicates diabetic retinopathy stabilization, and the index above 1.0 indicates retinopathy progression. The index can therefore be used to assess the effectiveness of treatment and to predict DR, which is essential for determining further patient management tactics.
References 1.International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: https://www.diabetesatlas.org 2.Pasyechnikova N., Moroz O. Study on the effect of quercetine and lipoate on lipid peroxidation in the retina in experimental diabetes. Oftalmologicheskii Zhurnal, 2016;(4):38–42. 3.Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab J. 2018;42:364-376. 4.Alekseev IB, Kochergin SA, Vorob'eva IV, Mikhaleva LG. On some pathogenic features of diabetic retinopathy in type II diabetes mellitus and the role of antioxidants and ginkgo biloba. Vestn Oftalmol. 2013; (3):89-93. 5.Kangilbaeva G, Jurabekova A. Effect of EGb 761 (Tanakan) Therapy in Eyes with Nonproliferative Diabetic Retinopathy. International Journal of Pharmaceutical Research. Jul - Dec 2020. Vol 12. Supplementary Issue 2, P. 3019-3023. 6.McKeage K, Lyseng-Williamson KA. Ginkgo biloba extract EGb 761® in the symptomatic treatment of mild-to-moderate dementia: a profile of its use. Drugs Ther Perspect. 2018;34(8):358-366. 7.Aznabaev BM, Gabdrahmanova AF, Muhamadeev TR, Gallyamova GR, Alexandrov AA. Ophthalmoneuroprotection in nonproliferative diabetic retinopathy and eye hemodynamics. RMJ Clinic ophthalmology. 2014; (2): 71-75. 8.Bakhritdinova F, Kangilbaeva G, Mirrakhimova S, Jurabekova A. Development of dopplerographic criteria for the progression and prognosis of diabetic retinopathy. European science review 2018;1-2:113-117. 9.Blum M, Eichhorn M, Vilser W. Haemodynamics and Diabetic Retinopathy. Klin. Augenheilk. 2005; 222(6):463-470. 10.Divya K, Kanagaraju V, Devanand B, Jeevamala C, Raghuram A, Sundar D. Evaluation of retrobulbar circulation in type 2 diabetic patients using color Doppler imaging. Indian J Ophthalmology. 2020;68:1108-1114. 11.J Fernando Arevalo. Classification of diabetic retinopathy and diabetic macular edema. World Journal of Diabetes. 2013; 4(6):290-294. 12.JK, Wang PW, Taylor S, Haskova Z. Durability of Diabetic Retinopathy Improvement with As-Needed Ranibizumab: Open-Label Extension of RIDE and RISE Studies. Ophthalmology. 2019;126(5):712-720. 13.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl.1):S84-S87 14.Javadzadeh A. The effect of posterior subtenon methylprednisolone acetate in the refractory diabetic macular edema. BMC Ophthalmol. 2016;(6):15-19. 15.Iwase T, Kobayashi M, Yamamoto K, Ra E, Terasaki H. Effects of photocoagulation on ocular blood flow in patients with severe non-proliferative diabetic retinopathy. PLoS One. 2017. 12 (3): e0174427. 16.Royle P, Mistry H, Auguste P. Pan-retinal photocoagulation and other forms of laser treatment and drug therapies for non-proliferative diabetic retinopathy: systematic review and economic evaluation. Health Technol Assess. 2015;19 (51):v-xxviii, 1-247 17.Filho JA, Messias A, Almeida FP, Ribeiro JA., Costa RA, Scott IU, et al. Panretinal photocoagulation ( PRP) versus PRP plus intravitreal ranibizumab for high-risk proliferative diabetic retinopathy. Acta Ophthalmol. 2011;89(7): e567-e572. 18.Kangilbaevaa G., Bakhritdinova F., Nabieva I., Jurabekova A. Eye hemodynamic data and biochemical parameters of the lacrimal fluid of patients with non-proliferative diabetic retinopathy. Data in Brief 2020; Volume 32: 106237. 19.Tanaka S, Tanaka S, Iimuro S, Yamashita H, Katayama S, Akanuma Y, et al. Predicting macro- and microvascular complications in type 2 diabetes: the Japan Diabetes Complications Study/the Japanese Elderly Diabetes Intervention Trial risk engine. Diabetes Care. 2013;36(5):1193-1199. 20.Haider S, Sadiq SN, Moore D, Price MJ, Nirantharakumar K. Prognostic prediction models for diabetic retinopathy progression: a systematic review. Eye. 2019;33: 702–713. 21.Tsao HY, Chan PY, Su EC. Predicting diabetic retinopathy and identifying interpretable biomedical features using machine learning algorithms. BMC Bioinformatics. 2018;19(Suppl 9):283. Published 2018 Aug 13.
No conflict of interest was declared by the authors
|