J.ophthalmol.(Ukraine).2021;4:79-85.
|
http://doi.org/10.31288/oftalmolzh202147985 Received: 23 February 2021; Published on-line: 16 August 2021 Clinical and morphological characteristic of the case of secondary enucleation of the eye with retinoblastoma N. F. Bobrova, T. A. Sorochynska, O. V. Artiomov, M. V. Bryn. SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) E-mail: filatov.detskoe7@gmail.com TO CITE THIS ARTICLE: Bobrova NF, Romanova TV, Artiomov OV, Brin MV. Clinical and morphological characteristic of the case of secondary enucleation of the eye with retinoblastoma. J.ophthalmol.(Ukraine). 2021;4:79-85. http://doi.org/10.31288/oftalmolzh202147985
Purpose: To analyze case of secondary enucleation of a child’s eye with recurrent retinoblastoma (RB) developing due to parents’ failure to take the child to surveillance appointments after salvage eye therapy. Material and Methods: Observation and treatment of a 10-month child diagnosed with bilateral RB. The right eye was diagnosed with RB at stage T1N0M0, and the left eye, with RB at stage T3cN0M0 complicated by secondary glaucoma. Results: Salvage eye therapy was performed which included six cycles of combined multiagent chemotherapy (with systemic intravitreal chemoreduction melphalan injection), followed by three courses of laser coagulation of tumor focus in the right eye and additional seventh local chemotherapy in the left eye. As a result, type 4 RB regression pattern in the right eye, and was developed incomplete type 2 RB regression pattern (“fish-flesh” appearance) in the left eye. However, after the child did not visit the medical care facility for surveillance and treatment during seven months, tumor regrowth developed. Secondary enucleation of the left eye was performed due to big tumor size, anterior chamber involvement blindness, and the absence of visual functions without prospects for their improvement. Histopathology found undifferentiated retinoblastoma with invasion into the episclera, the child was administered two cycles of chemoreduction as well as external beam radiotherapy of the left orbit using a medical linear accelerator system. Conclusion: Should tumor recurrence occur or if there is no tumor control, urgent secondary enucleation with eye ball histopathology and, if indicated, further adjuvant therapy are recommended to prevent metastasis and save child’s life. Keywords: retinoblastoma, retinoblastoma recurrence, continued growth, secondary enucleation, histopathological study
Introduction Retinoblstoma (RB) treatment aims at child S. survival. Globe salvage and preservation of vision are secondary goals. Enucleation rate for advanced unilateral RB has been reported to be as high as 66% to 91%, and for advanced bilateral RB, 17% to 43% [1], which is associated with late presentation. In recent years, advances in novel technologies have allowed to perform salvage eye treatment even in patients with advanced tumors. However, poor tumor response to treatment and tumor recurrence can lead to secondary enucleation in 9-17% of cases [2, 3]. That is why we report a case of secondary enucleation of a worse eye in bilateral retinoblastoma due to continued tumor growth after combined salvage eye therapy. Purpose: To analyse a case of secondary enucleation of a child’s eye with recurrent retinoblastoma developing due to parents’ failure to take the child to surveillance appointments after salvage eye therapy. Material and Methods A child S. lives in Dnipropetrovsk region and is being treated at the Filatov institute, the Ukrainian Center for Pediatric Ophthalmology. The parents had noticed an increased size of the left eye and a white pupillary reflex when the baby was three months. They, however, failed to seek for medical advice for six months thereafter. At the age of nine months, the baby was seen by a local ophthalmologist, diagnosed with retinoblastoma of the left eye, and re-ferred to the Filatov institute for treatment. On June 10, 2019, at presentation at the Filatov institute, the right eye had a visual acuity of 0.06, and the left eye, doubtful light perception as assessed by Teller’s cards. Examination under general anesthesia was performed that included tonometry, biomicroscopy, ultrasound biometry, and fundus imaging using a PanoCam system.
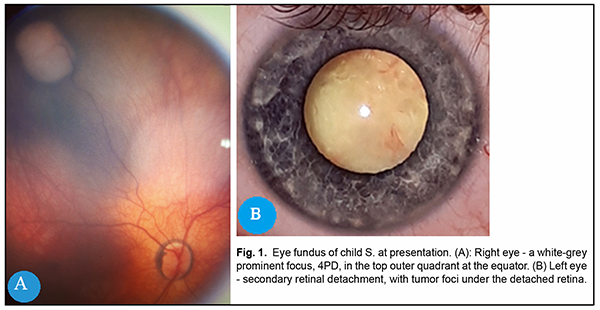
On examination the right eye, was quiestc and the media were transparent. Eye fundus showed a pigment ring around the papilla opticus, and a white-grey prominent tumor focus of about four disc diameters in the top outer quadrant at the equator, whereas the rest of the retina was unremarkable (Fig. 1A). On examination of the left eye, there was an exophoria of 15˚, macrophthalmos, anterior chamber shallow, mydriasis, the iris-lens diaphragm was anteriorly displaced, secondary retinal detachment behind the lens, with the posterior lens capsule contact at an outer half, and tumor foci in the internal portion of the detached retina (Fig. 1B). Intraocular pressure (IOP) of the RE was 18.0 mmHg and 23 mmHg in the LE. Table 1 presents ultra-sound biometry data.
Table 1. Ultrasound biometry data EyeAnterior chamber depth (mm)Lens thickness (mm)Axial length (mm) Right eye3.043.3420.72 Left eye1.643.3422.2
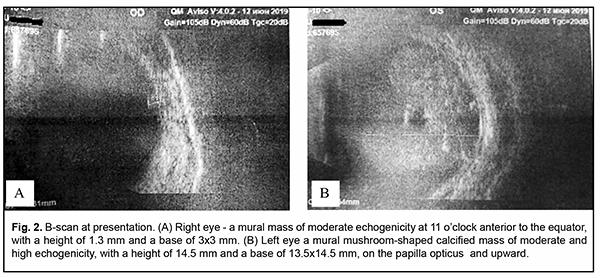
B-scan of the right eye showed a mural mass of moderate echogenicity at 11 o’clock posterior to the equator, with a height of 1.3 mm and a base of 3x3 mm (Fig. 2A). In the left eye, the iris root adhered to the posterior corneal surface along 0.9 mm in the top and bottom quadrants. B-scan of the left eye showed a mural mushroom-shaped calcified mass of moderate and high echogenicity, with a height of 14.5 mm and a base of 13.5x14.5 mm, on the papilla opticus and upward. (Fig. 2B).
MRI showed no focal changes in brain substance. Retrobulbar spaces were within normal limits, and no additional masses were detected. The left globe was subtotally filled with the neoplasm which showed ac-cumulation of the dye. The right eye was diagnosed with retinoblastoma at stage T1N0M0, and the left eye, with retinoblastoma at stage T3cN0M0 complicated by secondary glaucoma.
Results Given a bilateral tumor process, salvage eye therapy was performed which included six cycles of combined multiagent chemotherapy (by the method developed in the department - concurrent intravitreal melphalany and systemic chemoreduction [4]), followed by three courses of laser photocoagulation of tumor focus in the right eye and additional seventh local chemotherapy in the left eye. No complication was noted during the treatment period. Each cycle of therapy was preceded by a comprehensive examination of both eyes under general anesthesia. Patient’s examination on February 19, 2020, revealed the following.
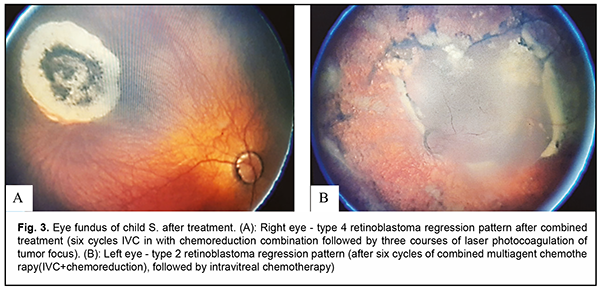
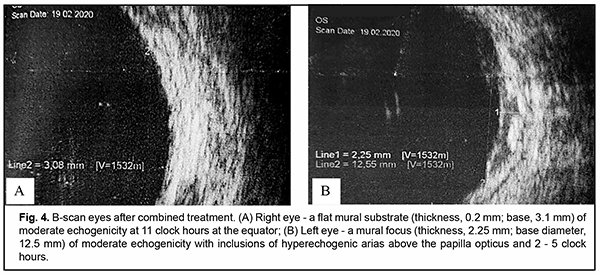
The right eye responded well to treatment, and had a visual acuity of 0.06 as assessed by Teller’s cards, and IOP of 14.0 mmHg. The eye was quiescent, the media were transparent, and a flat chorioretinal scar (thickness, 0.2 mm; base surface area, 3.1x3.1 mm, as assessed by B-scan) developed in the fundus at the site of tumor focus (Fig. 4A). The appearance was compatible with type 4 retinoblastoma regression pattern.
The treatment was less successful in the left eye due to a large initial size of the tumor. The left eye had a visual acuity of doubtful light perception, and IOP of 15.0 mmHg. Anterior chamber depth increased, the chamber was moderately deep, the pupil sluggish, vacuole-like inclusions and mild local haze appeared in the lens, and destruction of the vitreous body was observed. In the outer and lower segment of the eye, local retinal detachment was still present, and the fold detached from the posterior lens capsule. The left papilla optic disc was pale, a grayish translucent tumor focus retained upward and outward from the disc, a round gray focus (thickness, 2.25 mm; diameter, as large as 12.25 mm, as assessed by B-scan) was seen in the upper inner segment (Fig. 4b), with grey-yellow strip-shaped remnants of necrotic tissue nearby the focus, and small subretinal tumor seeds scattered across the retina (Fig. 3b). The appearance was compatible with incomplete type 2 retinoblastoma regression pattern (a homogeneous, translucent fish-flesh appearance). The child was recommended to have further treatment with an in-creased dose of intravitreal chemotherapy and further consolidation therapy. However, the child did not visit the institute during seven months, which the parents explained as being due to bans for intercity travels during quarantine associated with coronavirus COVID-19 pandemic. On examination on September 4, 2020, the right eye showed total fundus control.
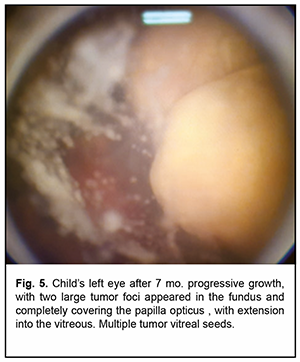
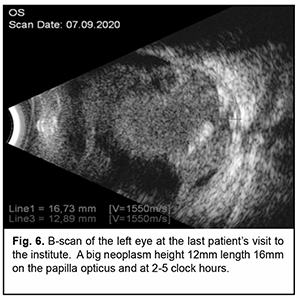
Left eye, visual acuity decreased to zero. Anterior chamber was shallow, flat peripheral anterior synechia with vessels formed in the outer half of the chamber, the pupil was sluggish, the lens showed vacuole-like inclusions and mild local haze, numerous tumor foci seedings appeared in the vitreous. Two large tumor foci appeared in the fundus and completely covered the papilla opticus, with extension into the vitreous (Fig. 5). B-scan showed that the iris attached to the cornea at 2 - 4 clock hours. Mural calcified mass of moderate and high echogenicity, with a height of 12 mm and a base of 13.5x14.5 mm, above the papilla opticus and running towards 2 - 5 clock hours from it (Fig. 6).
On September 8, 2020, on the basis of the findings of the last examination of the left eye (a large tumor involving two thirds of the vitreous and covering the papilla opticus, with numerous vitreal seedings and anterior chamber involvement, and the absence of visual functions without prospects for improvement), the left eye was enucleated using the method of high-frequency welding of biological tissues [5], a stump was formed by implanting an 18-mm intraocular insert Ekoflon, and placement of eye prosthesis was done. The surgery and postoperative period were uneventful.
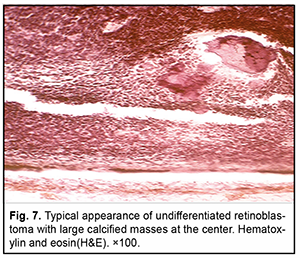
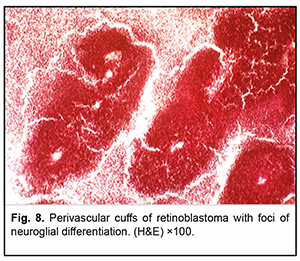
Histopathology found that the appearance of a large part of the tumor parenchyma was compatible with undifferentiated retinoblastoma (with foci of coagulative necrosis and calcified masses) (Fig. 7). Although in some sites, tumor tissue showed evidence of neuroglial differentiation, no true Flexner–Wintersteiner rosettes were observed (Fig. 8). The tumor node was as large as 12 mm, with its major mass located at the equator and not extending to the papilla opticus. However, due to tumor seeding, at some locations, the tumor displaced the retinal pigment epithelium and disseminated to the Bruch’s membrane, but without invasion of the choroid (Fig. 9).
On the basis of histopathology undifferentiated retinoblastoma with involvement of the episclera and without optic nerves invasion was diagnosed. There were multiple tumor cells implantation in the Bruch’s membrane, with Bruch’s membrane lesions at some locations. Due to episcleral invasion, the two cycles was prescribed as adjuvant chemotherapy of chemoreduction as well as external beam radiotherapy of the left orbit using a medical linear accelerator system (total dose of 50 Gy); both adjuvant chemotherapy and radiotherapy were received at the place of residence. The treatment was uneventful.
On December 12, 2020, a post-treatment examination found that the right-eye appearance was still compatible with type 4 retinoblastoma regression pattern. The patient had left eye anophthalmia. The conjunctival cavity was clear, and the “plus tissue” could not be palpated. The prosthesis in place, with satisfactory cosmetic effect. Stump mobility was good. The patient was recommended follow up at the institute every two months. Discussion Salvage eye therapy is commonly the first line of bilateral retinoblastoma treatment, and includes different types of chemotherapy [1, 6]: systemic intravenous chemoreduction (the CEV protocol) [7, 8], combined multiagent chemotherapy (intravitreal chemotherapy with systemic chemoreduction) [1, 4]; local chemotherapy; intraarterial chemotherapy (IAC) [3, 9, 10], paraocular chemotherapy [11, 12], intravitreal chemo-therapy (IVC) [1, 13, 14]. Systemic intravenous multiagent chemotherapy provides an adequate concentration of cytostatics in the peripheral blood, whereas IVC exerts a direct effect on the tumor (especially with endophytic growth) and massive retinal and vitreal RB cells dissemination [1, 9, 14, 15]. Sequential cycles of multiagent chemotherapy lead to reduced tumor size and enable to begin local fundus destruction, with indications for application of each method depending on the size of the tumor [7, 8]. Salvage eye therapy with the use of chemoreduction combined with consolidation therapy (laser photocoagulation, cryodestruction, brachytherapy) produced a tumor control rate of 60-76% [16-18], whereas IAC, rate of 74-91% [3, 19], and combined multiagent chemotherapy rate of 77.5% [1]. Most clinicians believe that type 1 (completely calcified tumor) and type 4 (flat chorioretinal atrophic scar) are most favorable regression types for prevention of tumor recurrence, because both these type indicate an inactive tumor, whereas type 2 ( “fish flesh” appearance without calcination), which was seen in our case, is difficult to differentiate from an active tumor and they most offen recapsed [1, 18, 20]. It is believed that the treatment of these eyes should be continued until there is evidence of regression type 1 or 4, indicating complete tumor regression [1, 19]. This is exemplified by the current case, when the parents’ failure to have the child’s treatment continued at regression type 2 resulted in disease recurrence in the form of continued tumor growth. Since salvare eye treatment is not always effective, surgeons had to do secondary enucleation in some cases[1, 16-19]. Pavlidou et al. have reported that secondary enucleation rate can be as high as 50% [21] due to poor tumor response to treatment, retinoblastoma recurrence and complications, ciliary body, iris and/or cornea invasion [1, 19, 21]. Fabian et al. have detected high-risk histopathologic features in 5 eyes (21%) after secondary enucleation [22]. All patients were treated with adjuvant systemic chemoreduction following enu-cleation. Neither deaths no metastases were recorded. Tumors in the vitreous were detected significantly more commonly in patients without high-risk histopathologic features, warranting meticulous clinical and histologic ex-aminations for this subset of patients [22]. Zhao et al. [23] reported that enucleation delayed longer than three months after diagnosis due to administration of neoadjuvant therapy was associated with mortality in four patients as a result of “down-staging” of disease and reduced surveillance leading to inappropriate management of high-risk disease. Brennan et al. report that [24], prolonged time of secondary enucleation was not associated with mortality. Several factors likely influenced this result. The majority of eyes were treated with initial chemoreduction and responded well to therapy. Late recurrence or progression of disease was treated with other modalities, in contrast to chemotherapy only, in the Zhao et al. [23] study. In addition, if patients underwent enucleation after chemotherapy, the original treatment plan was completed regardless of the histopathology to avoid “down-staging”. Patients with the longest time to enucleation for whom high-risk histopathology features were identified were appropriately treated with further adjuvant therapy. Finally, almost 90% in eyes Brennan et al. secondary enucleation group were enucleated within 30 days after diagnosis of disease progression [24]. Brennan et al. note the importance of a multimodal systemic approach toprogressive retinoblastoma treatment, with consideration of extent of disease prior to therapy, in time referral for enucleation when warranted, careful examination of histopathology, and appropriate post enucleation treatment to achieve recovery [23, 24]. Retinoblastoma is classified into two histological types, the undifferentiated type and differentiated type. The recurrence rate depends on the type of retinoblastoma differentiation, and is as high as 68% for poorly-differentiated tumors [20]. Choroidal invasion of retinoblastoma has been reported in 15-62% cases [1, 25, 26]. The risk of retinoblastoma metastasis depends on the extent of choroidal invasion, and it is estimated that metastasis is responsible for 11% to 81% of retinoblastoma deaths [27, 28]. Survival prognosis is much worse when choroidal invasion associated with any degree of optic nerve invasion, anterior uveal invasion and/or sclera invasion [26]. Choroidal invasion with scleral spreeding has been reported by Shields et al. in 43.2% of eyes with retinoblastoma after enucleation [26]. In reported case, histopathology study demonstrated that the large part of the tumor parenchyma was compatible with undifferentiated retinoblastoma (with foci of coagulative necrosis and calcified masses), and no true Flexner–Wintersteiner rosettes were observed. There was no tumor invasion into the optic nerve or choroid, in spite of disseminated tumor growth with implantation on the Bruch’s membrane. In addi-tion, retinoblastoma cells were found in the episclera, which is a significant risk factor for orbital involvement. Therefore, histopathology allowed us to estimate the risk of further tumor dissemination associated with uncontrolled growth and potential for choroidal involvement. Of note is the pattern of tumor growth presented in Fig. 9. This pattern, in contrast to major tumor mass presented in Figs. 7 and 8, shows no signs of degenerative changes and necrosis, indicating tumor growth towards the choroid, with the potential for choroidal invasion if enucleation is delayed. Beyond the above findings of histopathology and given the statistical data from the liter-ature, we can confidently assert that, in the current case, secondary enucleation with adjuvant therapy was necessary due to a threat to child’s survival. That`s why long follow up of the patient is necessary due to a high risk for tumor recurrence. Long term observation of patients with retinoblastoma is important if signs of high-risk tumor dis-semination are present. Since bilateral retinoblastomas frequently, demonstrate multicentric growth, constant monitoring is required to detect new tumor foci. The younger the child at the time of primary cancer treatment, the higher is the risk of development of another neoplasm. If early detected, the majority of cases of another neoplasm can undergo repeat eye-preserving treatment. This is the reason why, in bilateral retinoblastoma, the following surveillance strategy should be used: surveillance intervals of 1-3 months under general anesthesia until three years after completion of treatment, then intervals of 6 months until five years after completion of treatment, and annual surveillance intervals thereafter. Surveillance of a child with any form of retinoblastoma should not be discontinued. Conclusion
Treatment bilateral retinoblastoma depending on should be comprehensive, tumor stages in both eyes, and include systemic combined multiagent chemotherapy to prevent tumor generalization and achieve complete tumor control in both eyes. Type 2 retinoblastoma regression pattern (“fish-flesh” appearance) requires surveillance intervals as frequent as every 4-6 weeks and further combination therapy to prevent tumor recurrence. Finally, should tumor recurrence occur or if there is no tumor control, urgent secondary enucleation with histopathology and, if indicated, further adjuvant therapy are recommended to prevent metastasis and save child’s life. References 1.Bobrova NF, editor. Retinoblastoma. Odessa: Izdatelskii tsentr; 2020. Russian. 2.Ianchenko TV. [Clinical and epidemiological risks for retinoblastoma in Kemerovo region] [Abstract of thesis for the degree of Cand Sc (Med)]. Moscow; 2015. Russian. 3.Abramson DH, Daniels AB, Marr BP, et al. Intra-Arterial Chemotherapy (Ophthalmic Artery Chemosurgery) for Group D Reti-noblastoma. PLoS One. 2016 Jan 12;11(1):e0146582. doi: 10.1371/journal.pone.0146582. 4.Bobrova NF, Sorochynska TA. [Method of combined treatment for retinoblastoma]. Patent of Ukraine UA 55690U. issued on December 27, 2018. Ukrainian. 5.Bobrova NF, Vit VV, Sorochynska TA, Smaglii DV. [Method of the eye globe enucleation in retinoblastomas with a high risk of optic nerve invasion]. Patent of Ukraine UA 124022. Bulletin No.5/2018 issued on March 12, 2018. Ukrainian. 6.Shields CL, Fulco EM, Arias JD, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemo-therapy. Eye (Lond). 2013 Feb; 27(2): 253–64. doi: 10.1038/eye.2012.175. 7.Murphree AL, Villablanca JG, Deegan WF 3rd, et al. Chemotherapy plus local treatment in the management of intraocular reti-noblastoma. Arch Ophthalmol. 1996 Nov;114(11):1348-56. doi: 10.1001/archopht.1996.01100140548005. 8.Shields CL, De Potter P, Himelstein BP, et al. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Oph-thalmol. 1996 Nov;114(11):1330-8. doi: 10.1001/archopht.1996.01100140530002. 9.Francis JH, Roosipu N, Levin AM, et al. Current Treatment of Bilateral Retinoblastoma: The Impact of Intraarterial and Intravitre-ous Chemotherapy. Neoplasia. 2018 Aug; 20(8): 757–763.. doi: 10.1016/j.neo.2018.05.007. 10.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004 Apr;9(2):69-73. doi: 10.1007/s10147-004-0392-6. 11.Carcaboso A.M., Chiappetta D.A., Opezzo J.A. Episcleral implants for topotecan delivery to the posterior segment of the eye. In-vest Ophthalmol Vis Sci. 2010 Apr;51(4):2126-34. doi: 10.1167/iovs.09-4050. 12.Mallipatna AC, Dimaras H, Chan HS. Periocular topotecan for intraocular retinoblastoma. Arch Ophthalmol. 2011 Jun;129(6):738-45. doi: 10.1001/archophthalmol.2011.130. 13.Kaneko A, Suzuki S. Eye-Preservation Treatment of Retinoblastoma with Vitreous Seeding. Jap J Clin Oncol. 2003 Dec;33(12):601-7. 14.Munier FL, Gaillard MC, Soliman S, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibi-tion to conditional indications. Br J Ophthalmol. 2012 Aug;96(8):1078-83. doi: 10.1136/bjophthalmol-2011-301450. 15.Francis JH, Abramson DH, Gaillard MC, et al. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015 Jun;122(6):1173-9. doi: 10.1016/j.ophtha.2015.01.017. 16.Bartuma K, Pal N, Kosek S, et al. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Oph-thalmol. 2014 Aug;92(5):404-11. doi: 10.1111/aos.12282. 17.Ghose S, Nizamuddin SHM, Sethi A. Efficacy of induction chemotherapy in retinoblastoma, alone or combined with other adjuvant modalities. J Pediatr Ophthalmol. May-Jun 2002;39(3):143-50. 18.Shields CL, Mashayekhi A, Cater J, et al. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004. 2004;102:35-44; discussion 44-5. 19.Berry JL, Kogachi K, Murphree L, Jubran R, Kim JW. A Review of Recurrent Retinoblastoma: Children’s Hospital Los Angeles Classification and Treatment Guidelines. Int Ophthalmol Clin. Spring 2019;59(2):65-75. doi: 10.1097/IIO.0000000000000269. 20.Gündüz K, Günalp I, Yalçindağ N, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors lead-ing to eventual treatment with external beam radiotherapy and enucleation. Ophthalmol. 2004 Oct;111(10):1917-24. doi: 10.1016/j.ophtha.2004.04.016. 21.Pavlidou E, Burris C, Thaung C, et al. Anterior Segment Seeding in Eyes With Retinoblastoma Failing to Respond to Intraophthal-mic Artery Chemotherapy. JAMA Ophthalmol. 2015 Dec;133(12):1455-8. doi: 10.1001/jamaophthalmol.2015.2861. 22.Fabian ID, Stacey A, Chowdhury T et al. High-Risk Histopathology Features in Primary and Secondary Enucleated International Intraocular Retinoblastoma Classification Group D Eyes. Ophthalmology. 2017 Jun;124(6):851-858. doi: 10.1016/j.ophtha.2017.01.048. 23.Zhao J, Dimaras H, Massey C, et al. Pre-Enucleation Chemotherapy for Eyes Severely Affected by Retinoblastoma Masks Risk of Tumor Extension and Increases Death From Metastasis. J Clin Oncol. 2011 Mar 1;29(7):845-51. doi: 10.1200/JCO.2010.32.5332. 24.Brennan RC, Qaddoumi I, Billups C, et al. Comparison of High-Risk Histopathologic Features in Eyes with Primary or Secondary Enucleation for Retinoblastoma. Br J Ophthalmol. 2015 Oct;99(10):1366-1371. doi: 10.1136/bjophthalmol-2014-306364. 25.Kopelman JE, McLean IW, Rosenberg SH. Multivariate analysis of risk factors for metastasis in retinoblastoma treated by enuclea-tion. Ophthalmology. 1987 Apr;94(4):371-7. doi: 10.1016/s0161-6420(87)33436-0. 26.Shields CL, Shields JA, Baew KA. Choroidal invasion of retinoblastoma: metastatic potential and clinical risk factors. Br J Oph-thalmol. 1993 Sep;77(9):544-8. doi: 10.1136/bjo.77.9.544. 27.Eagle RC, Jr. High-Risk Features and Tumor Differentiation in Retinoblastoma. A Retrospective Histopathologic Study. Arch Pathol Lab Med. 2009 Aug;133(8):1203-9. doi: 10.5858/133.8.1203. 28.Kashyap S, Sethi S, Meel R, et al. A Histopathologic Analysis of Eyes Primarily Enucleated for Advanced Intraocular Retinoblas-toma from a Developing Country. Arch Pathol Lab Med. 2012 Feb;136(2):190-3. doi: 10.5858/arpa.2010-0759-OA.
|