J.ophthalmol.(Ukraine).2021;4:72-78.
|
http://doi.org/10.31288/oftalmolzh202147278 Received: 05 March 2021; Published on-line: 16 August 2021 Ultrastructural changes in the chorioretinal complex of the rat after inducing form-deprivation axial myopia only, diabetic retinopathy only and diabetic retinopathy in the presence of myopia I. M. Mykheitseva, N. I. Molchaniuk, Abdulhadi Muhammad, S. G. Kolomiichuk, O. O. Suprun SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine"; Odesa (Ukraine) TO CITE THIS ARTICLE: Mykheitseva IM, Molchaniuk NI, Muhammad Abdulhadi, Kolomiichuk SG, Suprun OO. Ultrastructural changes in the chorioretinal complex of the rat after inducing form-deprivation axial myopia only, diabetic retinopathy only and diabetic retinopathy in the presence of myopia. J.ophthalmol.(Ukraine). 2021;4:72-8. http://doi.org/10.31288/oftalmolzh202147278
Purpose: To determine the features of the microstructure of choreoretinal complex in rats with diabetes induced by streptozotocin in the presence of axial myopia. Material and Methods: Fifty-five Wistar rats (110 eyes; age, 2 weeks to 14 weeks) were used in experiments. Four groups were formed: group 1 (axial myopia only); group 2 (diabetes only); group 3 (both myopia and diabetes); and group 4 (controls; intact animals). High form-deprivation myopia was produced in two-week animals by surgically fusing the eyelids of both eyes and these animals were maintained under conditions of reduced illumination for two weeks to induce a more intense myopization of the globe. Eyelid sutures were removed on completion of these two weeks. Two weeks thereafter, type 2 diabetes mellitus was induced in rats with induced axial myopia and intact rats. A 50 mg/kg intraperitoneal streptozotocin injection for 5 days was used for this purpose. Elongated axial length and increased anterior chamber depth as measured by in vivo ultrasound were an objective criterion of the development of myopia in experimental animals. A glucose level of ≥ 4.5 mmol/L was a criterion of the development of diabetes. Two months after inducing diabetes, 14-week rats were sacrificed, and their eye tissue samples were processed by a routine method and assessed by electron microscopy. Ultrastructure of the choroid, RPE, and retinal photoreceptor cells were examined. Ultra-thin sections were cut, stained with lead citrate according to the procedure described by Reynolds, and observed with a PEM-100-01 Transmission Electron Microscope. Results: Our ultrastructural study found that myopization of the rat globe with elongation of the axial length somewhat reduced the severity of some ultrastructural changes in the choreoretinal complex in induced type 2 diabetes due to reduced choroidal swelling and dominance of compensatory processes with increased energy producing, protein synthesis and other functions in the endothelial vessels and choriocapillaries as well as RPE cells. Out findings seem to corroborate the concept that myopized eyes have capacity to somewhat buffer the development of severe diabetic retinopathy, likely due to some compensatory-and-restorative processes. Keywords: streptozotocin-induced diabetes, form-deprivation myopia, rats, electron microscopy, choroidal ultrastructure, retinal pigment epithelium, compensatory processes
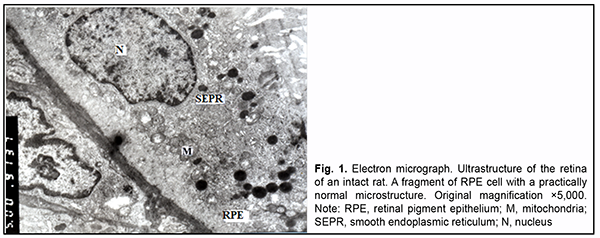
Introduction Diabetes mellitus (DM) is a multifactorial disease with pathological changes in numerous organs, including the eyes. Diabetic retinopathy (DR) is one of the most severe complications of DM. Clinical and experimental studies have determined the structural changes in the ocular tissues in DR. Vascular changes (vessel wall defects, reduced capillary filling, neovascularization, and glial and retinal neuronal damage [1]) may be considered major structural changes in the ocular tissues in DR. An experimental study by Zhdankina [2] noted that the pathomorphogenesis of the choreoretinal complex of the retina in rat models of retinopathy (e.g., diabetic retinopathy) was primarily associated with the development of microvascular changes and oxidative damage in the retina and choroid. The author believes that these factors are a trigger of degeneration of the chorioretinal complex in general and neurodegeneration in particular. In addition, dia-betes contributes to some features of the mechanisms of the development of structural changes in the retina and choroid. Thus, long-term non-compensated hyperglycemia induces retinal degeneration as a result of biochemical cascade. Initial endothelial damage with swelling and destruction of intracellular organelles and development of inflammatory responses in vessel walls is observed. This is followed by degeneration of pigment cells, massive deg-radation of neurosensory cell outer segment membranes, massive ganglion neuron death, and impaired interneu-ronal cooperation. Myopia is a common refractive error, and the global number of people with myopia has been steadily increas-ing. Particularly, the rate of high myopia has been increasing worldwide [3], and high myopia may cause some reti-nal complications. High myopia may contribute not only to retinal changes, but also to changes in the choroid, leading to chori-oretinopathy. The function of this ocular structure includes metabolism of the retinal pigment epithelium (RPE) and supplying blood to the outer retinal layers. In an OCT study by Barteselli and colleagues [4], regression analysis demonstrated that choroidal volume decreased with an increase in the axial length of the eye. Reduced choroidal thickness may lead to reduced supply of oxygen and nutrients to the retina. It has been demonstrated [5] that an increase in myopia resulted in significant thinning of the central choroid. The authors supposed that reduced choroidal volume resulting in altered blood flow may play an important role in complications of high myopia. Although numerous studies have been conducted on the pathogenesis and treatment of such a severe disease as diabetic retinopathy, there are still open questions in this area [6]. The morphological changes in the posterior eye in diabetes developing in the presence of high myopia are still to be elucidated. Concomitant diseases (particularly, eye diseases) are still an issue. Usually, in concomitant diseases, their symp-toms and course become more intense. Myopia in diabetes mellitus is somewhat paradoxic. Diabetics with high myopia rarely develop severe retinopathy, and practically do not exhibit proliferative DR [7, 8]. Changes in the op-tic disc and retinal neovascularization are less apparent and uncommon in myopic eyes of patients with diabetes mellitus [9]. In patients with monocular high myopia (-13.0 D), there were no signs of diabetic retinopathy in the myopic eye, whereas the emmetropic eye exhibited various stages of retinopathy, from severe non-proliferative retinopathy to proliferative retinopathy. Although the exact mechanism of this phenomenon is not clear, it has been reported that myopic eyes exhibited reduced blood flow, which was associated with less altered microcirculation in eyes of patients with diabetes melli-tus [7]. The purpose of the study was to determine the features of the microstructure of choreoretinal complex in rats with diabetes induced by streptozotocin in the presence of axial myopia. Material and Methods Fifty-five Wistar rats (110 eyes; age, 2 weeks to 14 weeks) were used in experiments. All manipulations were performed in compliance with the provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986) and General Ethical Principles for Experi-ments on Animals adopted by the 3rd National Congress (Kyiv, 2007). Deprivation myopia was produced in two-week animals by surgically fusing the eyelids of both eyes [10]. Thereafter, these animals were maintained under conditions of reduced illumination for two weeks to induce a more intense myopization of the globe [11]. Eyelid sutures were removed on completion of these two weeks. Two weeks thereafter, type 2 diabetes mellitus was induced in rats with induced axial myopia and intact rats. A 50 mg/kg intra-peritoneal streptozotocin injection for 5 days was used for this purpose [12]. The control group was composed of intact animals which were maintained under conditions of natural illumination. Therefore, four groups were formed: group 1 (axial myopia only, 30 eyes); group 2 (diabetes only, 30 eyes); group 3 (myopia and diabetes, 30 eyes); and group 4 (controls; intact animals; 20 eyes). Elongated axial length and increased anterior chamber depth as measured by in vivo ultrasound were an objective criterion of the development of myopia in experimental ani-mals. A glucose level of ≥ 4.5 mmol/L was a criterion of the development of diabetes. Two months after inducing diabetes, 14-week rats were sacrificed, and their eye tissue samples were assessed by electron microscopy. Ultra-structure of the choroid, RPE, and retinal photoreceptor cells were examined. Tissue samples were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.4), post-fixed with 1% osmium te-troxide in phosphate buffer (pH 7.4), dehydrated through an ascending ethanol series, and embedded in an Epon/Araldite mix. Thereafter, ultra-thin sections were cut, stained with lead citrate according to the procedure de-scribed by Reynolds, and observed with a PEM-100-01 Transmission Electron Microscope (Selmi, Sumy, Ukraine). Results In the choroid of intact rats, the interstitial loose connective tissue showed collagen fibers with a normal ultra-structure, and the ground substance and fibroblasts with various functional states. Lumens of large and medium-sized vessels were filled with red cells. Some of their endothelial cells showed normal ultrastructure, whereas other showed somewhat clear cytosol. In the choriocapillaris layer, lumens of some capillaries of were occluded by the endothelial nucleus at some sites, and endothelial cells with increased number of typical organelles were occasional-ly seen (Fig. 1).
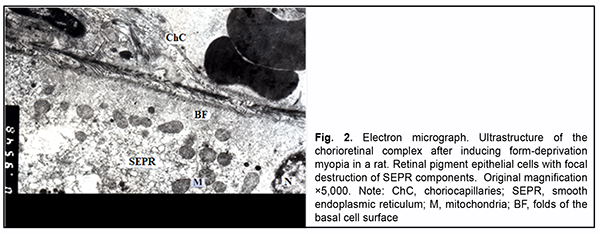
In the RPE, there were cells with various functional states. Some of them showed normal ultrastructure, i.e. components of smooth endoplasmic reticulum (SEPR), mitochondria and other typical organelles. Other RPE cells showed (1) moderate electron-density of cytosol with increased number of typical organelles or (2) somewhat clear cytosol, loose arrangement of the components of SEPR, small number of mitochondria, solitary large phagosomes, small lysosomes and residual corpuscles, possibly due to their increased functional loads. Nuclei of all cells were large and oval-shaped, had well-shaped nuclear pores and nucleolus, and their karyoplasm was of moderate elec-tron density. At the basal cell surface, folds were arranged irregularly: there were sites with long and tortuous folds, and others with short and even folds, and some other sites showed almost no folds. Apical microvilli were normal. Most photoreceptor cells had normal structure. In individual photoreceptor cells, outer segments contained not membranes but a vesicle-like structure. It is likely that this was the early stage of membrane formation. In addition, these cells had clear cytoplasm and dilated cisternae of the granular endoplasmic reticulum (GEPR). In rats with induced myopia, as opposed to intact rats, swelling of the ground substance and connective tissue cells were seen at some stromal sites. Endothelial cells of vessels and capillaries had cytoplasm either of an in-creased electron density in which ultrastructural components were poorly determined or with clear cytosol and or-ganelle swelling. In the choriocapillaris layer, lumens of individual capillaries were significantly reduced in size or completely obliterated by endothelial cell bodies. However, at some sites, the above choriocapillaris structures showed a normal ultrastructure. In the RPE layer, some cells showed signs of hydropic changes, with clear cytosol, swelling of inner mitochondri-al matrix with focal or complete destruction of mitochondrial cristae and dilated components of GEPR and SEPR. Other RPE cells had an electron-dense cytosol and swelling of cytoplasmic membrane organelles. Their cytoplasm also contained myelin-like coils formed as a result of disruption of cell membrane structures. RPE cells with normal ultrastructure were occasionally seen. The basal surface of RPE cells displayed short or tortuous folds, although these folds were practically absent at some sites, indicating impaired transport processes between the choriocapil-laris and RPE cells. At the apical surface of RPE cells, microvilli were thick or fragmented, and accumulation of photoreceptor outer segment debris was seen beneath RPE cells, indicating impaired phagocytosis in these cells. The debris showed pathological changes (Fig. 2).
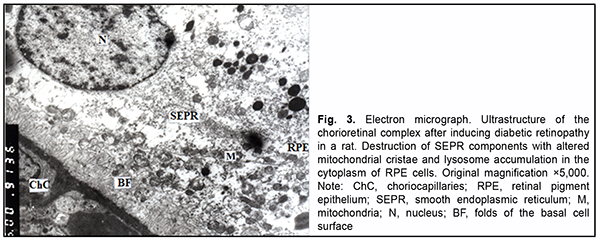
At the sites of contact with photoreceptor inner segments, photoreceptor outer segments showed a structure re-sembling that for intact animals, and the disconnection of membranes was seen only in some segments. The de-tachment of a part of the outer segment from the inner segment was occasionally seen. In addition, photoreceptor inner segments with some cisternae of the granular endoplasmic reticulum appeared dilated, destruction of cristae of individual mitochondria was seen, and some of them showed organelle destruction. The cytoplasm and nuclei of photoreceptors showed no remarkable structural changes. Swelling of Muller cell processes was observed in the area of photoreceptor nuclei. In rats with induced diabetic retinopathy, the connective tissue of the choroid showed a clear ground substance and loose collagen fibrils. Lumens of vessels and capillaries were filled with red cells. In addition, they were signifi-cantly reduced in size at some locations. The ultrastructure of endothelial cells of vessels and the choriocapillaris was heterogeneous: some cells showed a normal structure, whereas other showed signs of hydropic degeneration or compensatory-and-restorative processes, with increased amount of cytoplasmic organelles, large nucleus and dif-fuse chromatin in the nucleolus. It is noteworthy that fenestrations were well seen at the thinned cytoplasmic sites of endothelial cells of the choriocapillaris, and solitary microvilli were seen at the luminal surface of these cells. Polymorphic changes were noted in the RPE layer. Some RPE cells showed a practically normal structure, with exclusion of loosely arranged vesicles of SEPR and lysosome accumulation in the cytoplasm. Others showed signs of nuclear membrane and cytosol swelling and a reduced amount of organelles. Some cells had signs of organelle destruction, with total loss of cytoplasm at individual sites. The basal surfaces of RPE cells showed no folds at some sites, and either deep and even or tortuous and short folds at other sites. Their state reflected the activity of the pump function between these sites of RPE cells and endothelial cells of the choriocapillaris. The apical microvilli of RPE cells were focally fragmented (Fig. 3).
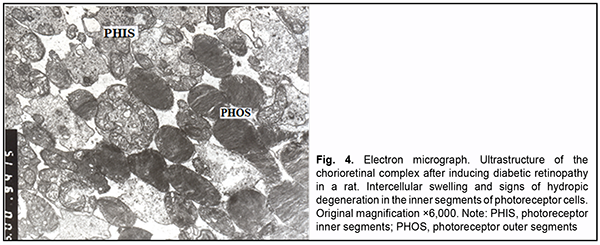
Intercellular swelling was noted in the photoreceptor cell layer. Photoreceptor outer segment debris with focal de-struction of disc membranes was accumulated beneath RPE cells. Disconnection of membranes was observed in the outer segments of photoreceptors. Mitochondrial pathology and dilated cisternae of the GEPR were seen in the inner segments. Swelling of the Goldgi apparatus and GEPR components was observed in the cytoplasm of photo-receptors (Fig. 4).
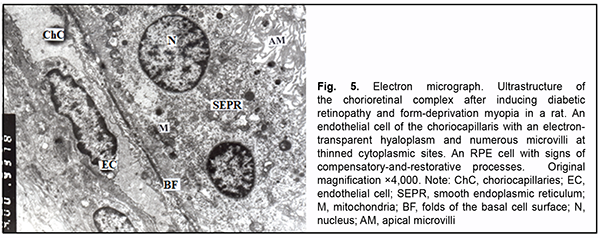
Signs of swelling of stromal components and endothelial cells of vessels and capillaries of the choriocapillaris were less frequently observed after inducing diabetic retinopathy with myopia. However, there were differences in the lumens of vessels and, especially, the choriocapillaris. At some sites, the lumens were dilated, electron-lucent, and included solitary red cells. At other sites, the lumens were of a normal size and plasma had an increased elec-tron density, or the lumens were of a significantly reduced size due to a large nuclear portion of endothelial cells. There were polymorphic changes in the RPE cells. Some RPE cells had signs of altered organelles, others had an increased number of organelles, which indicated compensatory-and-restorative processes. Each of the latter cells had two nuclei with dispersed chromatin, the nucleoli were seen at the nuclear membrane (which indicated active metabolic processes between the nucleus and cytoplasm), accumulation of polysomes was observed beneath the folds of the basal surface, and mitochondria with a normal structure were arranged around the nuclei and distribut-ed across the entire cytoplasm. This, in general, reflected increased energy producing, protein synthesis and other functions (Fig. 5). However, apical microvilli of some cells were commonly fragmented or destroyed, which pre-vented phagocytosis. In the area of photoreceptor cells, we noted extracellular swelling, focal destruction of indi-vidual outer segments, dilated cisternae of the GEPR, and pathology of some mitochondria in the inner segments and cytoplasm. Swelling was typical of Muller cell processes at the outer plasmatic membrane.
Discussion A protective effect of high myopia on the retina in diabetes has been extensively discussed in the ophthalmolog-ical literature, but the mechanisms of this effect have not been elucidated. It is not understood in which way myo-pia (particularly, high myopia) prevents the development of severe retinopathy and especially proliferative DR in diabetics [7-9]. Lim and colleagues [14] investigated the relationship between refractive error, ocular biometry, and DR, and concluded that myopic refraction and longer axial length are associated with a lower risk of complications in DR. Man and colleagues [15] concluded that, in persons with diabetes, eyes with longer axial lengths are less likely to have DR and diabetic macular edema. A meta-analysis by Fu and colleagues [16] confirmed that both myopic refraction and longer AL are associated with a lower risk of DR. All studies, however, noted that, the mechanisms of the protective effect myopia against the development of diabetic retinopathy have not been investigated. Some studies [17, 18] supposed that it is a reduced retinal blood flow that contributes to a reduction in the risk and severity of diabetic retinopathy in axial myopia. A study by Man and colleagues [19] investigated if reduced retinal capillary flow (RCF) is a potential mechanism underpinning a protective relationship between a longer axial length and a decreased risk of diabetic retinopathy. They found no association between axial length and RCF or between RCF and DR. Therefore, further research is required to determine the mechanisms underpinning the protec-tive effect of myopia against the development of diabetic retinopathy [20]. In the current experimental study, we aimed to determine the features of the microstructure of choreoretinal complex in rats with diabetes induced by streptozotocin in the presence of axial myopia. We found that swelling was typical of the ground substance of the connective tissue and of the endothelial cells of vessels and capillaries for the choriocapillaris of rats with diabetes induced by streptozotocin in the presence of axial myopia. Mild vacuolar degeneration was observed in RPE cells. Retinal photoreceptor cells were characterized by extracellular swelling, destruction of the disc membranes of individual outer segments and altered organelles of some inner segments. Signs of intra and extracellular swelling were more severe in rats with induced diabetic retinopathy in the chori-ocapillaris and RPE and photoreceptor cells than in rats with induced myopia. It is noteworthy that at the luminal surface of endothelial cells of the choriocapillaris there were solitary microvilli thickened at some sites, which in-creased the surface of these cells for penetration of nutrients from the lumens of the choriocapillaris to RPE cells. Stromal swelling was somewhat reduced after inducing diabetes in the presence of myopia. In the endothelial cells of vessels and the choriocapillaris, as well as in the RPE cells, signs of compensatory-and-restorative processes were more common than signs of hydropic degeneration. However, photoreceptor cells were characterized by signs of extracellular swelling and intracellularly altered organelles. Therefore, two months after inducing streptozotocin diabetes in the presence of axial myopia in rats, ultrastruc-tural study of the chorioretinal complex found changes in the structure of the endothelial cells of vessels and the choriocapillaris, RPE cells and photoreceptors, which were characteristic of early diabetes and early myopia. At the same time, the devastating changes in the ultrastructure of these cells after inducing diabetes in the presence of my-opization of the globe were somewhat retarded, likely due to some compensatory-and-restorative processes. The mechanisms of these processes should be, however, investigated. In conclusion, first, myopization of the rat globe with elongation of the axial length somewhat reduces the sever-ity of some ultrastructural changes in the choreoretinal complex in induced diabetic retinopathy due to reduced choroidal swelling and dominance of compensatory processes with increased energy producing, protein synthesis and other functions in the endothelial vessels and choriocapillaries as well as RPE cells. Second, the ultrastructural features that we found in the choreoretinal complex of rats with diabetes induced by streptozotocin in the presence of axial myopia suggest a capacity of myopized eyes to somewhat buffer the development of severe diabetic reti-nopathy, likely due to some compensatory-and-restorative processes.
References 1.Maltsev EV, Zborovska OV, Dorokhova AE. [Fundamental aspects of the development and treatment of diabetic retinopathy]. Ode-sa: Astroprint; 2018. Russian. 2.Zhdankina AA. [Morphological patterns of retinal changes in retinopathy of various origin and their correction with antioxidants: an experimental study]. Abstract of dissertation for the degree of Dr Sc (Med). Tomsk; 2013. Russian. 3.Avetisov ES. [Myopia]. Moscow: Meditsina; 2002. Russian. 4.Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012;119:2572–8. doi:10.1016/j.ophtha.2012.06.065. 5.Astakhov YuS, Belekhova SG. [Choroidal thickness in various grades of myopia]. Oftalmologicheskiie vedomosti. 2013;6(4):35-8. Russian. 6.Balashevich LI, Izmailov AS, editors. [Diabetic ophthalmopathy]. St. Petersburg: Chelovek; 2012. Russian. 7.Wang X, Tang L, Gao L, Yang Y, Cao D, Li Y. Myopia and diabetic retinopathy: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2016 Jan;111:1-9. 8.Bazzazi N, Akbarzadeh S, Yavarikia SM, et al. High myopia and diabetic retinopathy: A Contralateral Eye Study in Diabetic Pa-tients with High Myopic Anisometropia. Retina. 2017;37(7):1270–6. 9.Dujić M, Nikolić Lj, Misailović K, Ignjacev M. Occurrence of changes in the eye in diabetic retinopathy with significant myopia. Srp Arh Celok Lek. Nov-Dec 1998;126(11-12):457-60. Serbian.. 10.Beuerman RW, Saw S-M, Tan DTH, Wong T-Y, editors. Myopia: Animal Models to Clinical Trials. 1st ed. Toh Tuck Link (Sin-gapore): World Scientific Publishing Company Pte Ltd; 2010. 11.Mykheitseva IN, Abdulhadi Muhammad, Putienko AA, et al. [Inducing form-deprivation myopia in animals]. Oftalmol Zh. 2018;2:50-5. Russian. 12.Abdulhadi Muhammad, Mykheitseva IN, Putienko AA, et al. [Relationship of the axial length and anterior chamber depth of the rat globe and development of retinal abnormalities in type 2 diabetes mellitus with myopia]. Oftalmol Zh. 2018;6:44-50. Russian. 13.Reуnolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17(1):208-12. doi: 10.1083/jcb.17.1.208. 14.Lim LS, Lamoureux E, Saw SM. Are myopic eyes less likely to have diabetic retinopathy? Ophthalmology. 2010 Mar;117(3):524-30. doi: 10.1016/j.ophtha.2009.07.044. 15.Man RE, Sasongko МВ, Sanmugasundram S, et al. Longer Axial Length Is Protective of Diabetic Retinopathy and Macular Edema. Ophthalmology. 2012 Sep;119(9):1754-9. doi: 10.1016/j.ophtha.2012.03.021. 16.Fu Y, Geng D, Liu H, Che H. Myopia and/or longer axial length are protective against diabetic retinopathy: a meta-analysis. Acta Ophthalmol. 016 Jun;94(4):346-52. doi: 10.1111/aos.12908. 17.Dastiridou AI, Ginis Н, Tsilimbaris М. Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of axial length. Invest Ophthalmol Vis Sci. 2013 Mar 1;54(3):2087-92. doi: 10.1167/iovs.12-11576. 18.Shimada N, Ohno-Matsui К, Harino S. Reduction of retinal blood flow in high myopia. Graefes Arch Clin Exp Ophthalmol. 2004;242:284–8. doi: 10.1007/s00417-003-0836-0. 19.Man RE, Sasongko MB, Xie J, et al. Decreased Retinal Capillary Flow Is Not a Mediator of the Protective Myopia–Diabetic Reti-nopathy Relationship. Invest Ophthalmol Vis Sci. 2014 Sep 30;55(10):6901-7. doi: 10.1167/iovs.14-15137. 20.Man RE, Sasongko MB, Wang JJ, Lamoureux EL. Association between myopia and diabetic retinopathy: a review of observa-tional findings and potential mechanisms. Clin Exp Ophthalmol. 2013 Apr;41(3):293-301. doi: 10.1111/j.1442-9071.2012.02872.x. Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
|