J.ophthalmol.(Ukraine).2021;3:10-14.
|
http://doi.org/10.31288/oftalmolzh202131014 Received: 08 January 2021; Published on-line: 29 June 2021
Outcomes of aflibercept for post-vitrectomy vitreous hemorrhage in patients with proliferative diabetic retinopathy O. O. Putienko 1, D. M. Pogorilyi2, V. O. Putienko2 1 SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine»; Odesa (Ukraine) 2 Military Medical Clinical Center, Southern Region; Odesa (Ukraine) E-mail: alputienko@gmail.com TO CITE THIS ARTICLE:Putienko OO, Pogorilyi DM, Putienko VO. Outcomes of aflibercept for post-vitrectomy vitreous hemorrhage in patients with proliferative diabetic retinopathy. J.ophthalmol.(Ukraine).2021;3:10-14. http://doi.org/10.31288/oftalmolzh202131014 Background: The incidence of vitreous hemorrhage after vitrectomy for proliferative diabetic retinopathy (PDR) has been reported to vary from 21% to 80%. Purpose: To assess the efficacy of gas exchange tamponade (GET) in combination with intravitreal aflibercept for post-vitrectomy vitreous hemorrhage in patients with PDR. Material and Methods: We reviewed the medical records of 47 patients (47 eyes) who developed vitreous hemorrhage after vitrectomy for PDR and received a 20% C3F8 GET in combination with 2.0-mg intravitreal aflibercept (EYLEA®). Results: At 2 months, examination showed “transparent” vitreous cavity in 43 eyes (91.5%), and vitreous hemorrhage was still present in other 4 eyes (8.6%), including a recurrence in 2 eyes (4.3%). At 6 months, examination showed “transparent” vitreous cavity in 46 eyes (97.9%), and only one eye (2.1%) developed a recurrence of vitreous hemorrhage within two months to six months after primary surgery. At the 2 month follow-up, visual acuity was 0.1 to 0.3 in 24 eyes (51%), better than 0.35 in 17 eyes (36.2%), and lower than 0.1 in only 6 eyes (12.8%). At the 6 month follow-up, visual acuity remained similar to that at month 2, but the number of eyes with visual acuity better than 0.35 increased from 17 to 19. Conclusion: A 20% C3F8 GET in combination with intravitreal aflibercept is an effective method of treatment for vitreous hemorrhage after vitrectomy in patients with PDR and may be widely used in practice. Keywords: proliferative diabetic retinopathy, post-vitrectomy vitreous hemorrhage, gas exchange tamponade, aflibercept Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
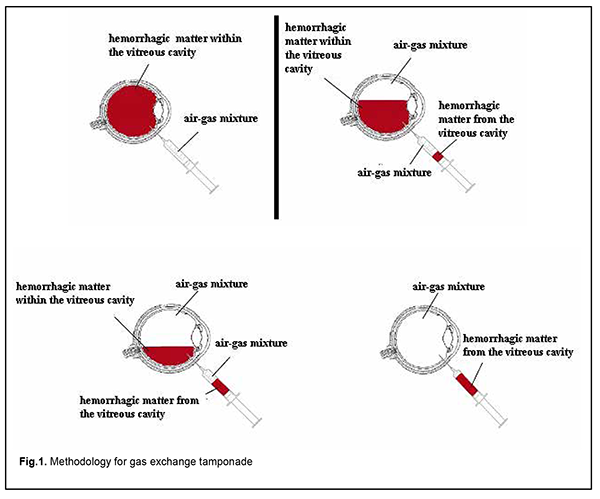
Introduction Proliferative diabetic retinopathy (PDR) is the major cause of blindness in the developed world [1]. Although there have been significant advances in the surgical treatment of PDR, a high incidence (up to 80%) of postoperative vitreous hemorrhage is still a major problem of this treatment [2-5]. The incidence of vitreous hemorrhage after vitrectomy for PDR has been reported to vary from 21% to 80% [3, 6-9]. Vitreous cavity hemorrhage which does not resolve spontaneously within two months exerts a toxic effect on the retina, and may result in ophthalmic hypertension and cataract progression, leading to chronic uveitis and other complications. Non-clearing postopertative vitreous cavity hemorrhage (POVCH) necessitates revision surgery in approximately a third to a half of those cases experiencing POVCH and approximately 10% of all patients undergoing surgery [4, 5, 10]. Patients with postoperative vitreous cavity hemorrhage have a delay in their visual recovery and, in as much as half of cases, this results in worse vision than was present preoperatively. Currently, vitreous hemorrhage after vitrectomy is treated by a revision surgery, either without or with vitreous tamponade with air, 20% sulfur hexafluoride (SF6) or 10% perfluoropropane (C3F8) [10-13]. Gas exchange tamponade (GET) is the simplest method of treatment for post-vitrectomy vitreous hemorrhage which aims to remove the non-transparent matter from the vitreous cavity, and tamponade the cavity with air alone or air-gas mixture. Recent studies have demonstrated that a 20% C3F8 GET was most effective, with a high surface tension of the gas bubble contributing to a longer tamponade effect on the retina compared to other gas mixtures or air [12, 14]. A continued proliferative process in the vitreous cavity (with the VEGF level in the vitreous cavity exceeding the norm in 84% patients with a recurrence of vitreous hemorrhage at 2 months after primary intravitreal surgery) was a major cause of hemorrhage in the presence of general decompensation [14]. These findings necessitate the use of anti-VEGF agents in revision surgeries for a substantial improvement in the efficacy of treatment. To our best knowledge, no study has been conducted on the use of GET in combination with aflibercept for post-vitrectomy vitreous hemorrhage in patients with PDR. The purpose of the study was to assess the efficacy of gas exchange tamponade in combination with intravitreal aflibercept for post-vitrectomy vitreous hemorrhage in patients with PDR. Material and Methods We reviewed the medical records of 47 patients (47 eyes) who developed vitreous hemorrhage after vitrectomy for PDR and received a 20% C3F8 GET in combination with 2.0-mg intravitreal aflibercept (EYLEA®). Informed consent for the surgery was obtained from all patients. The method of GET is as follows. The patient is placed in a sitting position. A needle is inserted 4 mm from the limbus at 6 o’clock to aspirate 4.0 to 4.5 mL of hemorrhagic matter. A mixture of C3F8 and air (20% C3F8) is prepared by drawing a 1.0-ml sample of pure C3F8 and adding a 4.0-ml sample of sterile air into a 5-ml syringe, and this mixture is injected into the ocular cavity. At conclusion, a 2.0-mg portion of aflibercept (EYLEA®) is injected 4 mm from the limbus at 6 o’clock. The scheme is presented in Figure 1.
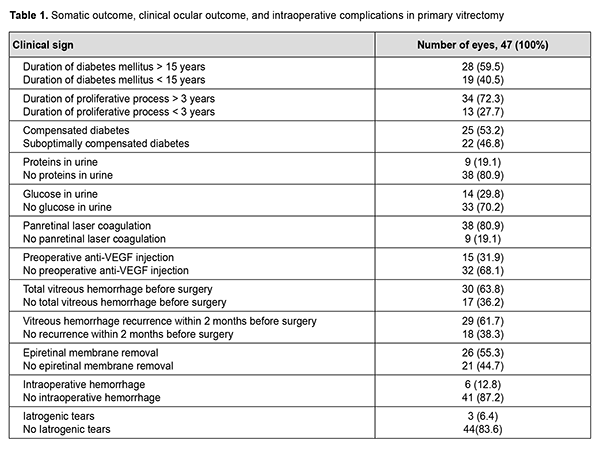
Indication for primary vitrectomy was intravitreal hemorrhage in the absence of epiretinal membrane in 22 eyes (46.8%), intravitreal hemorrhage in the presence of epiretinal membrane and tractional retinal detachment approaching or involving the macula in 23 eyes (48.9%), and combined tractional and rhegmatogenous retinal detachment in 2 eyes (4.3%). Table 1 presents the somatic outcome, clinical ocular outcome, intraoperative complications, and features of the tamponade and postoperative course of primary vitrectomy.
A 25-G vitrectomy was performed in a routine manner: after a subtotal vitrectomy, epiretinal membrane was removed as much as possible. In addition, sterile air was injected into the ocular cavity to flatten the retina, if required, and, panretinal laser coagulation and retinal tear endocoagulation, if retinal tears were present. Surgery was completed without vitreous tamponade in 2 eyes (4.3%), and by tamponade with air in 6 eyes (12.8%), 10% C3F8 in 7 eyes (16.7%), and 20% C3F8 in 32 eyes (68.1%). Visual outcomes ranged from light perception to 0.12, and, in 32 eyes (72.3%), were in the range of 0.01 to 0.05. At 2 months after primary intravitreal surgery (the time point of GET), visual acuity in most cases (42 eyes; 89.4%) was accurate light projection. Follow-up was performed at 2 months and 6 months. The chi-square test was used for statistical analysis. The study followed the ethical standards stated in the Declaration of Helsinki, the European Convention on Human Rights and Biomedicine and relevant laws of Ukraine. Results No complication was observed during GET in any case. Early after surgery, four eyes (8.5%) developed hyphema, which resolved with conservative therapy. In addition, exudative response was noted in 2 eyes (4.3%), which necessitated inflammatory therapy for resolution of fibrin. At 2 months, examination showed “transparent” vitreous cavity in 43 eyes (91.5%), and vitreous hemorrhage was still present in the rest of eyes (Table 2). Two eyes (4.3%) developed a recurrence of vitreous hemorrhage within two months after primary surgery, for which they received a 20% C3F8GET in combination with aflibercept.
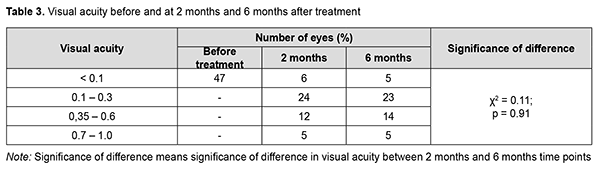
At 6 months, examination showed “transparent” vitreous cavity in 46 eyes (97.9%), and only one eye (2.1%) developed a recurrence of vitreous hemorrhage within two months to six months after primary surgery, for which it received a 20% C3F8 GET in combination with aflibercept. During this period, iris neovascularization with increased IOP was observed in 2 eyes (4.3%), which required hypotensive therapy with additional aflibercept. Visual acuity values at baseline and at 2 months and 6 months in study eyes are presented in Table 3. At the 2 month follow-up, visual acuity was 0.1 to 0.3 in most eyes (24 eyes; 51%), better than 0.35 in 17 eyes (36.2%), and lower than 0.1 in 6 eyes (12.8%; including 4 eyes with non-resolving vitreous hemorrhage). At the 6 month follow-up, visual acuity remained similar to that at month 2, but the number of eyes with visual acuity better than 0.35 increased from 17 to 19.
Discussion Applying anti-VEGF agents in eyes before vitrectomy for PDR is important for decreasing the rates of hemorrhagic complications during and early after surgery. However, the rates of post-vitrectomy vitreous hemorrhage in these patients were not lower than in those whose did not receive anti-VEGF therapy before surgery [3, 6, 8-10]. These findings also confirm the fact that, in spite of the surgery creating conditions for the stabilization of the proliferative process, a recurrence of hemorrhage after vitrectomy develops due to the progression of the proliferative process. Therefore, application of anti-VEGF therapy for vitreous hemorrhage after vitrectomy is relevant. Various approaches to the management of post-vitrectomy vitreous hemorrhage have been reported, including office-based air-fluid exchange, which is somewhat similar to our approach [2, 7, 8, 11]. The office-based air-fluid exchange technique [2] is performed through two ports: a 30-gauge needle, attached to a syringe filled with sterile air, is inserted superotermporally (non-dependent position) through the pars plana into the vitreous cavity; a second 30-gauge needle, attached to a 10-mL syringe, is inserted through the pars plana at 6 o’clock (dependent position) into the vitreous cavity. Injection of air through the nondependent syringe is performed, matching the rate of aspiration of the hemorrhage through the dependent syringe. However, those authors do not perform injection of an anti-VEGF agent. Behrens and colleagues [2] found their technique to be rather efficacious, with 3 of 16 eyes (19%) requiring a repeat vitrectomy. That is, in their study, the rate of hemorrhage recurrence was substantially higher than in the current study. It is noteworthy that, in their study [2], the rate of postoperative complications was high, with these complications including, particularly, hypotony in 1 eye, elevated IOP in 1 eye (6.7%), and recurrence of tractional retinal detachment in 3 eyes (20.1%), which was also substantially higher than in the current study. A rather high rate of recurrence of vitreous hemorrhage at later (> 4 months) time points after vitrectomy is currently a major issue [9, 10, 15]. In the current study, the rate of recurrence in the period from 2 months to 6 months was not high, and was even lower than during the first two months after primary vitrectomy. It is likely that this is associated with the effect of aflibercept, which allowed stabilizing the progression of proliferative process and avoiding a high rate of hemorrhage. Currently, intravitreal anti-VEGF injection as a monotherapy is considered as a treatment of post-vitrectomy vitreous hemorrhage, with only few reports with small samples on this subject in the literature. Chatziralli and colleagues [6] compared intravitreal ranibizumab treatment (n = 19) and pars plana vitrectomy (PPV; n = 18) in patients with recurrent vitreous hemorrhage due to PDR, who were previously treated with PPV. At month the two groups did not differ regarding the change in visual acuity or the rate of recurrent vitreous hemorrhage [6]. We have demonstrated previously that the rate of recurrence of vitreous hemorrhage was significantly lower in PDR patients treated for vitreous hemorrhage with a 20% C3F8 GET in combination with ranibizumab than in those treated with a 20% C3F8 GET only [12]. We believe that application of anti-VEGF monotherapy for vitreous hemorrhage after vitrectomy warrants further research with larger samples sizes. Therefore, we demonstrated that gas exchange tamponade with 20% C3F8 in combination with intravitreal aflibercept was the most effective method of treatment for vitreous hemorrhage after vitrectomy in patients with PDR, with stable treatment outcomes in 97.9% of cases and as low rate of recurrence of vitreous hemorrhage as 2.1% at 6 months. References 1.Abbate M, Cravedi P, Iliev I. Prevention and treatment of diabetic retinopathy: evidence from clinical trials and perspectives. Curr Diabetes Rev. 2011 May;7(3):190-200. 2.Behrens AW, Uwaydat SH, Hardin JS, Sallam AB. Office-based Air-Fluid Exchange for Diabetic Postoperative Vitreous Cavity Hemorrhage. Med Hypothesis Discov Innov Ophthalmol. Summer 2019;8(2):104-109. 3.Berk Ergun S, Toklu Y, Cakmak HB, Raza S, Simsek S. The effect of intravitreal bevacizumab as a pretreatment of vitrectomy for diabetic vitreous hemorrhage on recurrent hemorrhage. Semin Ophthalmol. 2015 May;30(3):177-80. 4.Steel DHW, Connor A, Habib MS. Entry site treatment to prevent late recurrent postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2010 Sep;94(9):1219-25. 5.Shi L, Yi-Fei H. Postvitrectomy diabetic vitreous hemorrhage in proliferative diabetic retinopathy. J Res Med Sci. 2012 Sep;17(9):865-71. 6.Chatziralli I, Dimitriou E, Theodossiadis G, Bourouki E, Bagli E, Kitsos G, Theodossiadis P. Intravitreal ranibizumab versus vitrectomy for recurrent vitreous haemorrhage after pars plana vitrectomy for proliferative diabetic retinopathy: a prospective study. Int Ophthalmol. 2020 Apr;40(4):841-847. 7.Khuthaila MK, Hsu J, Chiang A, DeCroos FC, Milder EA, Setlur V, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol. 2013 Apr;155(4):757-63, 763.e1-2. 8.Sato T, Morita S, Bando H. Early vitreous hemorrhage after vitrectomy with preoperative intravitreal bevacizumab for proliferative diabetic retinopathy. Middle East Afr J Ophthalmol. Jan-Mar 2013;20(1):51-5. 9.Tsubota K, Usui Y, Wakabayashi Y, Suzuki J, Ueda S, Goto H. Effectiveness of prophylactic intravitreal bevacizumab injection to proliferative diabetic retinopathy patients with elevated preoperative intraocular VEGF in preventing complications after vitrectomy. Clin Ophthalmol. 2019 Jun 28;13:1063-1070. 10.Yang CM, Yeh PT, Yang CH, Chen MS. Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am J Ophthalmol. 2008 Aug;146(2):211-217. 11.Nosov SV. [Tactics of treatment for late post-vitrectomy vitreous hemorrhage in patients with diabetes mellitus]. Oftalmokhirurgiia. 2011;3:53-6. Russian. 12.Putienko AA, Elhaj Ali, Pogorelyi DN. [Results of treatment of post-vitrectomy vitreous hemorrhage in patients with proliferative diabetic retinopathy]. Oftalmol Zh. 2015;2:22-6. Russian. 13.Koutsandrea CN, Apostolopoulos MN, Chatzoulis DZ, et al. Hemostatic effects of SF6 after diabetic vitrectomy for vitreous hemorrhage. Acta Ophthalmol Scand. 2001 Feb;79(1):34-8. 14.Putienko AA, Pogorelyi DN. [cytokine profile of blood and vitreous fluid in proliferative diabetic retinopathy patients with vitreous hemorrhage after vitrectomy]. Kazanskii meditsinskii zhurnal. 2013;94(1):26-30. Russian. 15.Mahalingam P, Topiwalla TT, Ganesan G. Vitreous rebleed following sutureless vitrectomy: incidence and risk factors. Indian J Ophthalmol. 2018 Apr;66(4):558-561.
|