J.ophthalmol.(Ukraine).2021;3:70-74.
|
http://doi.org/10.31288/oftalmolzh202137074 Received: 16 April 2021; Published on-line: 29 June 2021 Complicated uveal cataracts after intrauterine viral infection: a case report N. F. Bobrova, T. V. Romanova, A. V. Shilik SI "The Filatov Institute of Eye Diseases and Tissue Therapy of the NAMS of Ukraine; Odesa (Ukraine) E-mail: filatov.detskoe7@gmail.com TO CITE THIS ARTICLE:Bobrova NF, Romanova TV, Shilik AV. Complicated uveal cataracts after intrauterine viral infection: a case report. J.ophthalmol.(Ukraine).2021;3:70-74. http://doi.org/10.31288/oftalmolzh202137074 We report a case of complicated binocular cataract caused by intrauterine uveitis of mixed viral etiology (herpes simplex virus 1/2, cytomegalovirus and Epstein-Barr virus), and describe the management algorithm, surgical treatment features and treatment outcome. Keywords: complicated cataract, intrauterine TORCH infections Conflict of Interest Statement: The authors declare no conflict of interest which could influence their opinions on the subject or the materials presented in the manuscript.
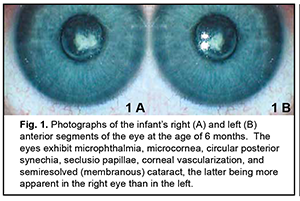
Intrauterine infections (IUI) are a group of fetal and neonatal inflammatory infectious diseases caused by various agents, with infections occurring during the antenatal or intranatal period. The incidence of intrauterine infections among newborns and infants of a few months old has been reported to be as high as 10-15%, and the role of these infections in neonatal diseases, disability and mortality has been demonstrated [1, 2]. An intrauterine infection is a common cause of active neonatal ocular inflammatory diseases (such as conjunctivitis, corneal ulceration, and endophthalmitis) and especially residual changes after these diseases (corneal opacity, atypical cataract, posterior synechia, choroidal foci, microphthalmia, and anophthalmia) [3-6]. The incidence rate of registered congenital infections in neonates has been increasing in recent years due to both an increase in the incidence rate of actual congenital infections and advanced diagnostics. The role of intracellular infections associated with uveitis in the pathogenesis of inflammatory process has been increasingly investigated recently [6-8]. Uveitis of mixed viral etiology can be especially challenging to diagnose and treat [9-11]. Diagnosis and prompt treatment of complications of intrauterine ocular infections is important in neonatal and eye care. The purpose of this paper is to report a case of complicated binocular cataract caused by intrauterine uveitis of mixed viral etiology in a child from Moldova. Material and Methods A child presented at the age of three months in 2019, was diagnosed with sequelae of intrauterine uveitis of mixed viral etiology (herpes simplex virus 1/2, cytomegalovirus and Epstein-Barr virus), and has been under our supervision since that date. The primary diagnosis was bilateral microphthalmia, microcornea, corneal vascularization, circular posterior synechia, seclusio papillae, and semiresolved (membranous) cataract (Fig. 1A, B). In addition, there was secondary ocular hypertension, nystagmus, and descending optic subatrophy.
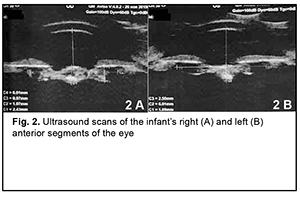
The secondary diagnosis was residual effects of congenital intrauterine infection, impaired cerebrospinal fluid dynamics, severely delayed statokinetic and psychoemotional development, functional fetal communications, open oval windows and CNS lesion due to intrauterine hypoxia. Findings from the history: The child was born at term (at 39 weeks of gestation) with a birth weight of 2890 g by a young healthy mother after her first planned pregnancy, and had no significant family history. Visual acuity was light perception OU. Examination under anesthesia: Intraocular pressure (IOP) was 27.0 mmHg OD and 25.0 mmHg OS. Keratometry was 43.0 D OD and 44.5 D OS. A-scan ultrasound biometry showed an axial length of 15.74 mm, an anterior chamber depth of 2.4 mm, and a lens thickness of 1.3 mm OD. The patient's left eye had an axial eye length of 14.68 mm, an anterior chamber depth of 1.6 mm, and a lens thickness of 1.9 mm. A-scan ultrasound of the right eye showed an anterior chamber depth of 2.4 mm and the non-homogeneous lens looking like a membrane which was thickened at the center and beneath the iris (Fig. 2 A). In addition, it showed a lens thickness of 1.1 mm and a lens horizontal diameter of 6.0 mm. The vitreous exhibited individual punctal-and-fibrous structures of low echogenicity. The retina appeared attached. Temporally to the optic disc, there was excavation of the vitreoretinal contour (excavation depth, 0.5 mm; excavation length, 6.0 mm), a posterior staphyloma.
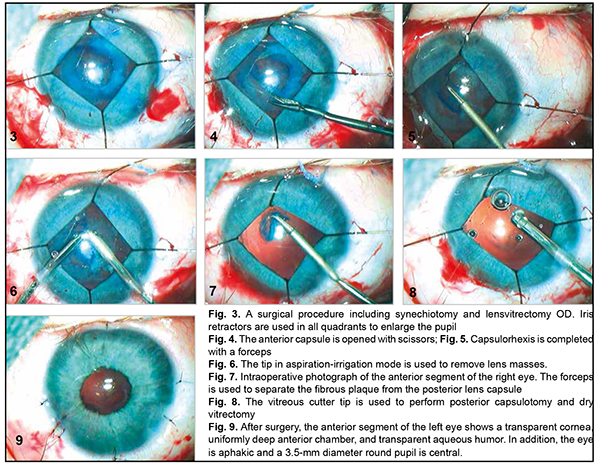
A-scan ultrasound of the left eye showed an anterior chamber depth of 2.5 mm and the non-homogeneous lens looking like a membrane which was thickened at the center and beneath the iris. In addition, it showed a lens thickness of 1.1 mm and a lens horizontal diameter of 6.0 mm (Fig. 2 B). The vitreous exhibited individual punctal-and-fibrous structures of low echogenicity. The retina appeared attached. Temporally to the optic disc, there was excavation of the vitreoretinal contour (excavation depth, 0.4 mm; excavation length, 5.5 mm), a posterior staphyloma. TORCH infection enzyme immunoassays: The child was found to be infected with cytomegalovirus (IgG antibodies, 11.5 IU/ml), herpes simplex virus 1/2 (IgG antibodies, 8.74 IU/ml), and Epstein-Barr virus (EBV capsid IgG antibodies, 18.92 IU/ml; EBV nuclear IgG antibodies, 17.74 IU/ml), whereas the mother, with cytomegalovirus (IgG antibodies, 34.1 IU/ml) and herpes simplex virus 1/2 (IgG antibodies, 46.7 IU/ml). Given the physical findings and general state of the child, the first treatment stage included anti-inflammatory and antibacterial therapy as follows: bilateral eye drops of okomistyn, four times daily; azopt, twice daily; maxitrol, four times daily; 0.15 ml parabulbar dexamethasone injection, №4. In addition, the infant was given 1.5-ml cefix, once daily orally, and laktiale, one sachet daily, for five days. The infant was seen and treated by the neonatologist, infection specialist and neurologist at the place of residence. Given low titers of IgG antibodies specific for herpes viruses (cytomegalovirus, herpes simplex virus 1/2, and Epstein-Barr virus), and the absence of clinical manifestations of infection, the infection specialist believed there was no need for specific antiviral therapy. Three months later, the infant’s general state improved, and he underwent re-examination under anesthesia. The clinical condition of the eyes improved. In addition, there was no change in visual acuity. The IOP decreased to 15.0 mmHg OD and 17.0 mmHg OS. The axial length as assessed by ultrasound A-scan biometry improved to 16.26 mm OD and 15.72 mm OD. Ultrasound scanning of both eyes showed the same changes as previously. Given that there was no vision in both eyes, on June 11, 2020, the right eye underwent surgery which included synechiotomy and lensectomy of the semiresolved cataract with anterior vitrectomy and basal iridectomy. Sub-tenon Kenalog was administered. The surgery was performed using a previously described method (Patent of Ukraine No. 69898 issued 10.05.2012, Bobrova and colleagues) [12] which included a translimbal approach using a bimanual technique, with 0.8-mm microincisions at 2 o’clock and 12 o’clock. Mesaton 1% was introduced into the anterior chamber through the paracentesis, but the pupil did not dilate due to posterior synechiae. Viscoat (a dispersive viscoelastic agent) was introduced into the anterior chamber. A basal iridectomy was performed at 6 o’clock position. A transection of the posterior circular synechia was done using a mictospatula, and the viscoelastic agent was additionally injected to prevent hemorrhage from iris vessels. The pupil dilated to 3.0 mm. After the viscoelastic agent was additionally injected, additional paracenteses were made at the 4, 7 and 9 o'clock positions, and iris retractors were placed to enable pupil dilation and better visualization of the lens (Fig. 3). The lens was found to be semiresolved, unevenly opaque (showing more apparent, severe and almost calcified opacity centrally and translucent masses peripherally), and exhibited fibrotic anterior capsule adhered centrally to the posterior capsule. The anterior capsule was stained with trypan blue. Thereafter, the anterior capsule was opened with a needle and scissors (Fig. 4), some Viscoat was injected into the capsular bag, the capsular adhesion was transected; an anterior capsulorhexis not larger than 4.5 mm was performed using a capsulorhexis forceps (Fig. 5). The tip in aspiration-irrigation mode was used to remove lens masses (Fig. 6). With this done, the posterior capsule exhibited a 3-mm fibrotic plaque centrally, some Viscoat was injected into the capsular bag, the plaque was separated from the posterior capsule (Fig. 7), and the latter was found to be unevenly opaque centrally. The vitreous cutter tip was used to perform a posterior capsulorhexis in the optic zone (Fig. 8) and partial anterior vitrectomy. With a contact lens put on the cornea, fundus examination of the eye showed a pale optic disc with clear margins. In addition, the eye showed no macular reflex, the vessels were narrow, and no focal changes were found. The iris retractors were removed, a miotic agent was used to dilate the pupil, and BSS plus, antibiotic (Zinacef) and sterile air were introduced into the anterior chamber. Surgical incisions were closed with 10-0 interrupted sutures (Fig. 9). Subconjunctival diprospan injection was administered.
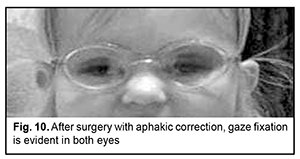
An identical procedure was performed in the fellow eye three months later, on September 15, 2020. Results In the presence of routine topical a general anti-inflammatory therapy combined with corticosteroids (topical maxidex and intravenous dexason), both eyes had no complications during the postoperative period. In both eyes, after surgery, visual acuity improved, and gaze fixation with ocular tracking response with afakic correction (OU +10.0 D) appeared. The child’s ocular tracking response is still preserved. At three months, the eyes were quiet and aphakic; the cornea was transparent, anterior chamber moderately deep, and aqueous humor transparent; a 3.5-mm diameter round pupil was central; the iris was moderately dystrophic, its pattern blurred, and no vascularization was observed. Ophthalmoscopic examination showed pale optic discs with clear margins, narrow vessels, no macular reflex, and no focal changes. The IOP by palpation was normal. Best-corrected visual acuity was pattern vision OU (Fig. 10).
The infant was referred to a neurologist. Discussion The mother is the source of infection in the fetus in an overwhelming majority of cases. The use of invasive techniques for the prenatal diagnostics and treatment (amniocentesis, umbilical cord puncture, etc.) and intrauterine transfusion of blood products through the umbilical cord (transfusion of red blood cell mass to the fetus in hemolytic disease) in the presence of failure of aseptic precautions, and prolonged pregnancy in the presence of early rupture of the amniotic membranes predispose to iatrogenic intrauterine infection. In antenatal infection, etiology is commonly viral (cytomegalovirus, rubella, coxsackievirus, etc.) or that of toxoplasma or mycoplasma, and vertical infection transmission can occur transovarially, transplacentally, or through the ascending pathways. The term “intrauterine infection”, when used as a diagnosis in clinical practice, should be accompanied by details regarding not only etiology, but also the period of infection and features of lesions of various internal organs. The term “TORCH syndrome” is also used to denote intrauterine infections. TORCH is an acronym composed of the first letters of the words Toxoplasmosis, Other (syphilis, listeriosis, viral hepatitis, chlamydiosis, HIV, mycoplasmosis, etc), Rubella, Cytomegalovirus (CMV), and Herpes infections. Direct and indirect studies should be performed to verify the disease if there is history or clinical features suggestive of intrauterine infection in the newborn. The direct diagnostic methods include virusological, bacteriological and molecular biology methods (polymerase chain reaction (PCR), DNA hybridization) and immune fluorescence. Of the indirect diagnostic methods, the most commonly used are enzyme immunoassays. The PCR has become increasingly popular recently as a method for detecting the agent in biological material. Any biological medium of the body (such as umbilical blood, saliva, urine, tracheal and oropharyngeal washes, conjunctival and urethral swabs, etc.) can be used as study material. The detection of specific IgM and low-avidity specific IgG antibodies along with longitudinal increases in their titers can be used for the verification of active intrauterine infection. In the case reported here, TORCH infection studies were performed, and the mother and the child were found enzyme immunoassay positive for CMV, HSV 1/2, and EBV, which provided evidence for intrauterine infection with these viruses. Complicated cataract is the most common ocular complication in patients with intrauterine infection. Surgical treatment of complicated cataract is the only treatment allowing restoration of vision in a child with this pathology [4, 6, 13, 14]. The choice of surgical procedure option for eyes with complicated cataract in children with sequelae of intrauterine infections is important because restoration of vision and reduction in the progression of inflammatory and trophic processes depend on this choice [13, 15, 16]. The surgical options available for the extraction of complicated uveal cataract (anterior phacoemulsification, pars plana lensectomy, combined corneal and pars plana approach, additional manipulations on the pupil, etc.) are not always adequately effective and may be followed by apparent postoperative inflammatory responses and such complications as conjunctival edema and hyperemia, corneal edema, inflammatory infiltration in the anterior chamber, iris edema, hyphema, synechia, and elevated IOP [6, 13, 17, 18]. Late after cataract surgery in the uveitic eye, in the presence of recurrent inflammation, recurrent adhesion of the pupillary margin of the iris may occur, causing iris bulging, obstruction of the anterior chamber angle and the development of secondary glaucoma [18-20]. Many prefer using an anterior corneal approach (1) since it enables a vast amount of reconstructive manipulations in the presence of obstruction of the pupil, when visualization of the complicated cataract is impossible, and (2) for dissection of the pre-lens films and synechiae [20]. It has been advocated to remove the posterior lens capsule only if it is apparently opaque. In such cases, the removal is combined with anterior pars plana vitrectomy, which is performed a certain period of time after primary surgery [15]. We believe that this method of surgery for complicated cataracts is disadvantageous, because (1) vitrectomy is rarely required in uveitic eyes with complicated cataract, and (2) the posterior lens capsule is preserved in most cases, which may cause such serious postoperative complications as the development of iridolenticular adhesion and obstruction of the pupil with iris bulging and the development of intraocular hypertension. A posterior approach to surgery for complicated cataract in uveitic eyes (the removal of the complicated cataract through the pars plana ciliaris approach) is used mostly in partial complicated cataracts, in the presence of apparent vitreous opacities (these opacities mostly occur in peripheral uveitis). This method enables dissecting the adhesion of the lens capsule to the hyaloid membrane and removing inflammatory products from the vitreous [6, 13, 17, 18]. However, given the fact that, in uveitis, the ciliary body is in the state of chronic inflammation, an intraoperative trauma to the ciliary body will affect the postoperative course, resulting in the requirement for massive and prolonged therapy. In addition, an intraoperative trauma to the ciliary body may impair its secretory function, with a decrease in aqueous secretion, resulting in the development of ocular hypotony and subatrophy. A combination method for removing complicated cataract in children was proposed by Kerimov and colleagues [15] and Foster and Barrett [14]. The method involves optical reconstructive surgery with anterior cataract extraction which is in some cases followed by pars plana vitrectomy. We believe that a disadvantage of this method is that anterior vitrectomy is performed not in each case of surgical treatment for complicated cataract, although it is vitrectomy that is considered an important element for preventing the recurrence of uveitis. The major disadvantage is the use of two surgical approaches, which increases the risk of intraoperative trauma. The method of Bobrova [12] for surgical treatment of complicated uveal cataract comprises a comprehensive reconstructive surgery through a translimbal approach using a bimanual technique and collet tools, and includes synechia dissection, removal of pre-lens films, performance of anterior and posterior capsulorhexis, irrigation and aspiration of complicated cataract, and partial vitrectomy. An advantage of the method is reduced intraoperative trauma to the eye, with the entire surgery performed through the anterior limbal approach to the anterior and posterior segments of the eye. Generally, the method reduces the risk of intraoperative and postoperative complications in the eye (especially in the pediatric age group which is associated with incomplete organogenesis) with complicated cataract associated with chronic uveitis. The case reported here is interesting in that, given the features of the clinical picture of intrauterine infection, an adequate treatment algorithm (conservative treatment followed by surgical treatment) was selected, enabling the stabilization of the preoperative IOP in both eyes and allowing to obtain a positive treatment outcome and to restore the transparency of the media to allow normal vision to develop. Conclusion Intrauterine viral infection (HSV 1/2, СМV, ЕВV) results in the development of severe ocular complications (microphthalmia, microcornea, complicated cataract, seclusio papillae, congenital glaucoma, vitreous opacities, and descending optic subatrophy) in children. Advanced reconstructive surgical treatment for complicated cataract through a translimbal approach using a bimanual technique and advanced collet tools reduces trauma to the cornea and ciliary body, with the entire surgery performed through the anterior limbal approach to the anterior and posterior segments of the eye, which not only enables the restoration of optic media transparency, but also provides comprehensive morphofunctional rehabilitation in infants.
References 1.Korneva MIu, Korovina NA, Zaplatnikov AL, etc. [Health status of intrauterine infected children]. Rossiiskii vestnik perinatologii i pediatrii. 2005;2:48-52. Russian. 2.Lago EG, Rodrigues LC, Fiori RM, Stein AT. Congenital syphilis: identification of two distinct profiles of maternal characteristics associated with risk. Sex Transm Dis. 2004 Jan;31(1):33-7. 3.Bobrova NF. [Surgical treatment of cataract in children]. In: [Veselovskaia ZF, editor. Cataract]. Kyiv: Knyha plus; 2002. p.173-202. Russian. 4.Bobrova NF, Skripnichenko ZM. [Toxic, congenital, and secondary cataracts]. Odessa: Feniks; 2017. 5.Katznelson AB. [Developmental abnormalities and ocular diseases in children younger than three years]. Leningrad: Medgiz; 1957. Russian. 6.Katargina LA, Khvatova AV. [Endogenous uveitis in children and adolescents]. Moscow: Meditsina; 2000. Russian. 7.Litvinenko AG, Ivanova NV. [Diagnostic features of the etiology of endogenous uveitis]. In: [Ophthalmology at the turn of the century: a collection of science works]. St. Petersburg; 2001. p.372. Russian. 8.Panova IE. [Clinical and immunological features of mixed ocular infections]. In: [Proceedings of the conference on the current issues of ocular infectious diseases]. 14 October 1999, Ufa, Russia. p.48-9. Russian. 9.Andzhelov VO, Krichevskaia GI, Slepova OS, et al. [Importance of persistent viral infections in the etiopathogenesis of endogenous uveitis in children: A guide for physicians]. Moscow; 1996. Russian. 10.Kaliberdina AF, Teplinskaia DE, Rozanova EB. [Cases of herpetic and cytomegalovirus lesions of the eye]. Vestn Oftalmol. 2003 Nov-Dec;119(6):43-4. Russian. 11.Krichevskaia GI, Andzhelov VO, Katargina LA, et al. [Reactivation of persistent herpes virus infection as a factor of endogenous uveitis in children]. Vestn Oftalmol. Mar-Apr 2005;121(2):22-4. Russian. 12.Bobrova NF, Dembovetska HM, Romanova TV, Nesterets OL. [Method of surgical treatment of complicated cataract in uveitis in children and adolescents]. Patent of Ukraine UA 69898 U; 2012. Ukrainian. 13.Takhchidi KhP, Shilovskikh OV. [A method of surgical treatment of complicated cataract in recurrent uveitis]. Patent for invention No. 2229273. Bulletin No. 15 issued 27.05.2004. Ekaterenburg. 14.Foster CS, Barrett F. Cataract development and cataract surgery in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Ophthalmology. 1993 Jun;100(6):809-17. 15.Kerimov KT, Mamedova TM. [Optical reconstructive surgery in complicated uveitis in children]. In: [Proceedings of the conference on the current issues of pediatric ophthalmology]. 29-31 October 2002, Moscow. p.118-20. Russian. 16.Shilovskikh OV, Samofalova OV, Ivanova MV. [A method of surgical treatment of cataract in chronic smoldering uveitis]. In: [Proceedings of the 5th Eurasian conference on ocular surgery]. 27-29 April 2009, Ekaterenburg, Russia. p.330. Russian. 17.Kanski J. Clinical Ophthalmology. 4th ed. Boston (MA): Butterworth- Heinemann; 1999. 18.Quiñones K, Cervantes-Castañeda RA, Hynes AY, et al. Outcomes of cataract surgery in children with chronic uveitis. J Cataract Refract Surg. 2009 Apr;35(4):725-31. 19.Dvali ML, Gabashvili TT, Beradze II. [Features of cataract surgery in children and adolescents]. Vestn Oftalmol. 2002 Mar-Apr;118(2):40-1. Russian. 20.Jancevski M, Foster CS. Cataracts and Uveitis. Curr Opin Ophthalmol. 2010 Jan;21(1):10-4.
|